Despite recent advances in endovascular therapies, the treatment of calcified popliteal artery lesions represents an ongoing challenge. Drug-coated balloons (DCBs), while effective in soft atherosclerotic lesions, are unable to expand completely in calcified lesions and the calcium presents a barrier to drug uptake.
Calcium also remains a challenge to traditional and interwoven self-expanding stents, resulting in stent malapposition and high rates of stent fracture.1–3 There are also biomechanical forces of external compression, torsion and elongation that occur with locomotion, which are unique to the popliteal segment.
Atherectomy aims to reduce the complications of traditional angioplasty such as dissection, recoil and disruption of the elastic lamina, which can result in smooth muscle cell proliferation and restenosis. The Stealth 360 Peripheral Orbital Atherectomy System (OAS; Cardiovascular Systems, Inc.) modifies calcified plaques using a 10 micron diamond-coated crown.4 The system uses the principle of centrifugal force, producing 360° of contact with calcified lesions to create a smooth concentric lumen by differential sanding.
The aim of this article is to assess the performance of the OAS in combination with percutaneous transluminal angioplasty with a DCB (PTA-DCB) on calcified popliteal artery lesions.
Method
From November 2018 to November 2019, six cases of focal popliteal arterial disease, involving the second popliteal artery segment (P2) were treated with OAS in combination with PTA-DCB. The Stealth 360 Peripheral OAS was used in each case. Patients were included if they had Rutherford classification (RC) stage III peripheral arterial disease with isolated calcified occlusions (according to the Fanelli classification) involving the popliteal artery and having at least two patent runoff arteries.5
Patients were excluded if they had thrombotic lesions detected using CT angiography, or MRI or duplex ultrasound for those with chronic kidney disease, in-stent or bypass restenosis or occlusions, if they had been treated with other atherectomy systems or cutting balloon angioplasty and if they had additional atherosclerotic lesions of the superficial femoral artery or the infrapopliteal segment.
Due to the retrospective design of the study, no ethical approval was necessary. We developed the paper’s protocol in accordance with the Declaration of Helsinki and all patients gave informed consent.
Procedure
All the patients had lesions in the P2 popliteal artery segment and two patients also had lesions in the first segment of the popliteal artery (P1). All patients were treated with a simple antiplatelet regimen of aspirin before the procedure. After revascularisation, heparin infusion was administrated for the first 24 hours (15 IU at 1.2 ml/h). Two months of dual antiplatelet therapy of 100 mg acetylsalicylic acid and 75 mg of clopidogrel were taken followed by lifelong monotherapy with acetylsalicylic acid. Under local anaesthesia, a diagnostic digital subtraction angiography of the affected limb was performed to identify the lesion and calcification. After catheterisation of the lesions, patients were treated with the OAS followed by PTA-DCB. We performed two passes per lesion with a 2 mm solid crown (the 1.5 mm, 1.75 mm solid crown, classic or micro crown were not available at our centre at the time) at 60, 90 and 120 rpm (a total of six passes per lesion) without the need for embolic protection. Thereafter, angioplasty was performed with a 5 mm diameter DCB with an average balloon inflation pressure of 8.7 ±1.6 atm for 3 minutes. A vascular closure device (Angioseal, St Jude Medical) was used for groin closure in all cases.
Endpoints
The primary endpoint was the primary patency rate at 6 months, defined as the freedom from haemodynamically significant stenosis (peak systolic velocity ratio ≥2.5) on duplex ultrasound at the target lesion and without target lesion revascularisation (TLR). The secondary endpoints were: the technical success, defined as the recanalisation of the occluded popliteal artery with residual angiographic stenosis <40% in absence of recoil, arterial dissection or perforation or distal embolisation without bailout stenting; the clinical success, defined as the improvement of the ankle brachial pressure index (ABPI) and one or more stages of RC at 6-month follow-up; 30-day morbidity, defined as the presence of any procedure-related complication at the puncture site, haematomas or pseudoaneurysms after the procedure or other clinical events; and the mortality rate at 6-month follow-up. Follow-up protocol included examination with ABPI and duplex ultrasound at 6 months postoperatively.
Results
Patient Demographics and Lesion Characteristics
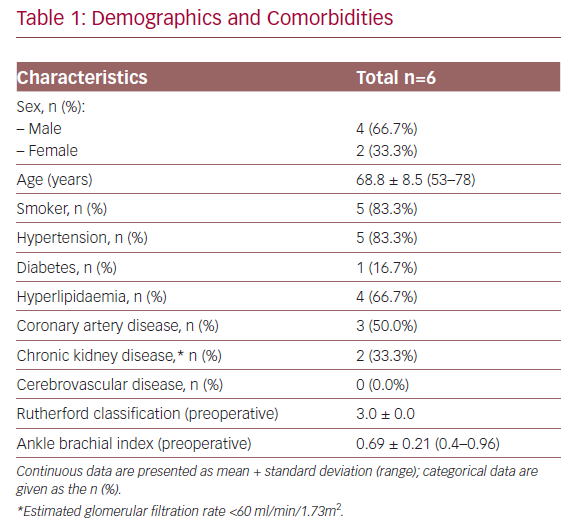
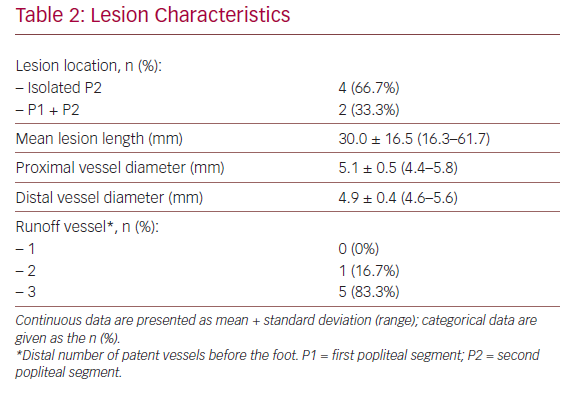
Baseline characteristics of the patients and their lesions are described in Tables 1 and 2. The mean age was 68.8 years (53.0–78.0) and four of the patients were men. In all cases, the patients presented with RC stage III severe claudication and a mean preoperative ABPI of 0.69 ± 0.21 (0.40–0.96). The mean lesion length was 30 ± 16.5 mm (16.3–61.7) and five of the patients (83.3%) had three distal patent vessels.
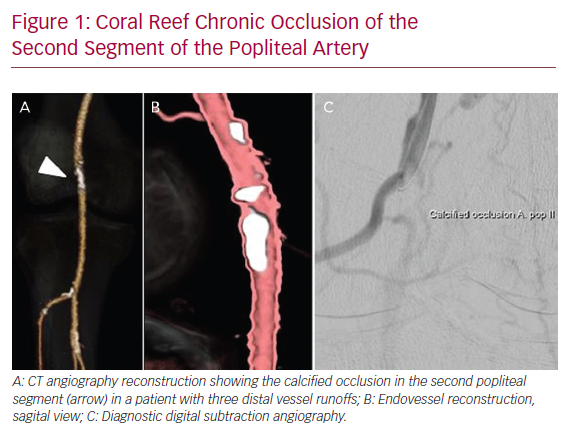
Figure 1 illustrates an example of a patient presenting with a severely calcified chronic occlusion of the popliteal artery and is representative of the lesions treated in this series.
Procedural Results and Clinical Outcomes
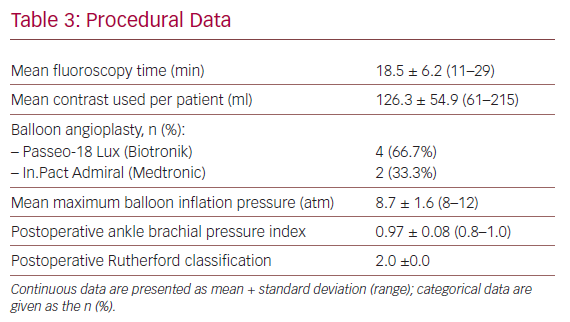
Table 3 shows details regarding devices used and the intraoperative variables. The most frequently used DCB catheter was the Passeo-18 Lux (Biotronik).
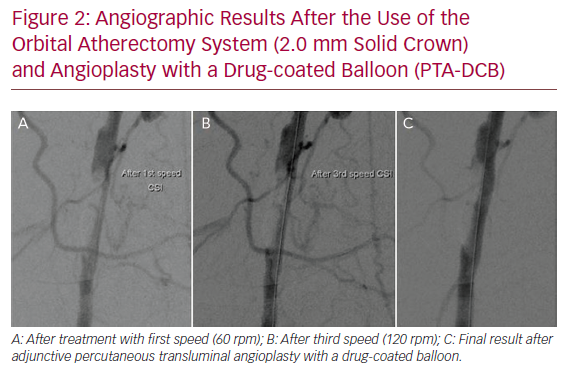
No distal embolisation, arterial dissection or perforation, puncture site haematomas or pseudoaneurysms were observed. The technical success rate was 100%, with no need for bailout stent placement. Figure 2 demonstrates the angiographic results after the use of the OAS and PTA-DCB.
In one case, spasm of the anterior tibial artery at ankle level was noted distally after treatment of a 28 mm lesion. The spasm was resolved without sequalae after local nitroglycerine administration (0.2 mg).
The postoperative mean ABPI was 0.97 ± 0.08 (0.8–1.0). Mean follow-up was 7.0 ± 4.2 months (1–12 months). During this period, there were no deaths, major amputations or TLR. Primary patency rate of the popliteal arteries was 100%.
Safety
One patient with history of MI and a triple coronary bypass more than 10 years before the procedure developed de novo AF in the first postoperative day and an asymptomatic elevation of ST interval in the ECG with new cardiac ischaemia proved in a myocardial scintigraphy. He was discharged after treatment adjustment by cardiology with control in 4 weeks to schedule coronary angiography.
Discussion
To our knowledge, this is the first paper focusing on the performance of the OAS in combination with PTA-DCB for isolated popliteal artery occlusions. The technical success rate was 100%. We observed no incidents of distal embolisation, arterial dissection, perforation or aneurysmal degeneration. No filter devices were used during the procedure. An improvement of ABPI and symptoms were observed in all patients postoperatively. The primary patency rate was 100% at 6 months.
Foley et al. reported the results of using the OAS to treat femoropopliteal disease. The treated cohort included 10 cases with lesions of the popliteal artery.6 However, due to the mixed population and presentation of the results for the entire femoropopliteal segment without specifying the performance of the technique in the popliteal segment, the study does not allow us to draw robust conclusions.
Orbital atherectomy is based on two principles: the centrifugal force due to the orbital motion produces 360° of contact of the crown with the vessel wall and creates a smooth concentric lumen, allowing the radius of orbit to increase with speed while maintaining continuous blood flow during treatment; and differential sanding with 30 micron diamond coating that sands away arterial calcium from atherosclerotic tissue, producing the elastic healthy tissue flexion minimising the coated surface on healthy tissue and liberation of micro-particulates of 2 microns. There is a low risk of distal embolisation without the need of filter devices. Both principles can explain the lower risk per se of perforation and embolisation when using OAS compared with other atherectomy systems.
In the COroNary CT Angiography Evaluation For Clinical Outcomes: An InteRnational Multicenter Registry (CONFIRM) series involving 3,135 patients, low rates of dissections (11.3% overall, 1.8% flow-limiting), embolism (2.2%) and vessel perforation (0.7%) were obtained. The goal after using OAS is to modify the vessel compliance rather than luminal gain.7 The absence of any relevant dissection in our cases despite the severe calcification of the lesions confirms the smooth modification of the plaque by OAS increasing the adaptability of the DCB. This avoids deployment of additional stents in an area which is associated with stent fractures due to high mechanical stress, external compression and elongation.1,8
Other atherectomy systems have also shown promising results, but the majority of them focused on the treatment of femoropopliteal lesions in general.9 Our group reported on the directional atherectomy with antirestenotic therapy (DAART) versus DCB angioplasty for isolated popliteal lesions.10 In that study, 41 patients were treated with DAART, including the TurboHawk atherectomy catheter (Medtronic), the SilverHawk (Medtronic) peripheral plaque excision system and the HawkOne atherectomy device (Medtronic); and the Pantheris optical coherence tomography atherectomy catheter (Avinger) and 31 with DCB angioplasty only. The results showed a higher technical success rate (93% versus 84%, p=0.24) and primary patency rate at 6 months with DAART (95% versus 79%). However, 7% of the treated cases resulted in an aneurysmal degeneration and 5% had an injury of the popliteal artery. Compared with those atherectomy systems, OAS was not associated with aneurysmal degeneration or with injury of the popliteal artery. One other advantage is that due to the mode of action, use of filter devices is not necessary, especially when a good distal vessel runoff is present.
Study Limitations
This is a single-centre report with a small number of patients and short-term follow-up. Nevertheless, isolated calcified popliteal lesions remain a significant challenge to treat and OAS is a relatively new technique in Europe. There is no comparison arm in the present study.
Conclusion
This is the first article focusing on the popliteal artery that evaluates the performance of PTA-DCB-assisted OAS. Short-term results are promising, with a low rate of major adverse events and no need for additional stent placement. OAS and PTA-DCB should be considered an alternative treatment option in selected cases, especially in younger patients, minimising the risk of additional deployment of stents. More data are needed to confirm these results.