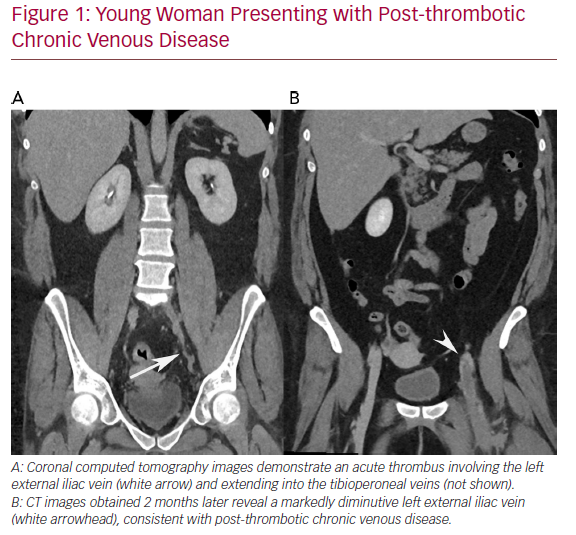
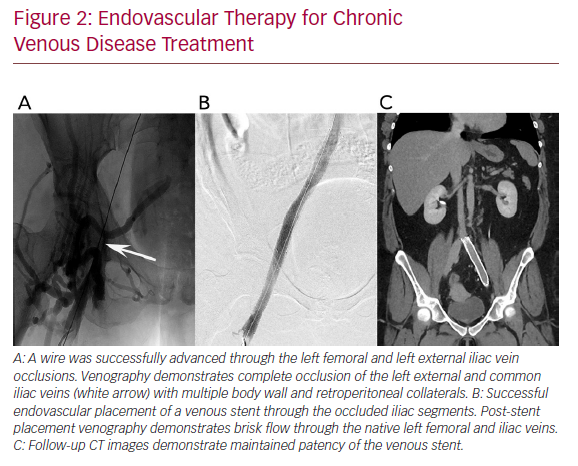
Chronic deep venous disease (CVD) affects millions of patients and causes significant morbidity, including lower extremity oedema, venous claudication, and in severe cases, venous ulceration. Commonly, CVD is caused by either thrombotic aetiologies, such as deep venous thrombosis (DVT), or by non-thrombotic aetiologies, such as in iliac vein compression syndrome (May−Thurner syndrome). In CVD, the affected veins become markedly atretic, thus impairing appropriate venous drainage, despite the formation of collaterals (Figure 1). Recently, endovascular intervention with percutaneous transluminal angioplasty and venous stent placement has become a mainstay treatment for this disease entity, and has been shown to have high rates of technical and clinical success (Figure 2).
A feared complication of venous stent placement is post-procedural in-stent restenosis and/or stent thrombosis. Previous studies have cited rates as high as 28% at 1 year and up to 62% at 5 years.1,2 An appropriate post-stent placement anticoagulation regimen is of utmost importance in the clinical management of these patients to maintain patency and provide durable symptom resolution. Several other factors are also essential for stent failure prevention, such as elimination of thrombus when treating acute DVT, appropriate stent landing and positioning into disease-free segments of the vein, and ensuring that inflow and outflow of the stent is optimised and sufficient.
Despite increasing rates of venous stent placement, few studies have been performed to inform the optimal antithrombotic therapy regimen, and no high-grade, evidence-based guidelines exist for the management of these patients. Most current practices reflect prior experiences in treating venous thromboembolism or are based on data produced from arterial stent placement. However, the pathophysiology underlying venous stent stenosis is distinct to its arterial counterpart, with animal models showing significantly different rates of intimal proliferation, hyperplasia and in-stent stenosis.3 These differences are likely related to markedly different flow dynamics, shear forces and vessel characteristics.4–6
In this article, we provide a review of the current data regarding anticoagulation therapy after venous stent placement and summarise currently practiced management.
Literature Review
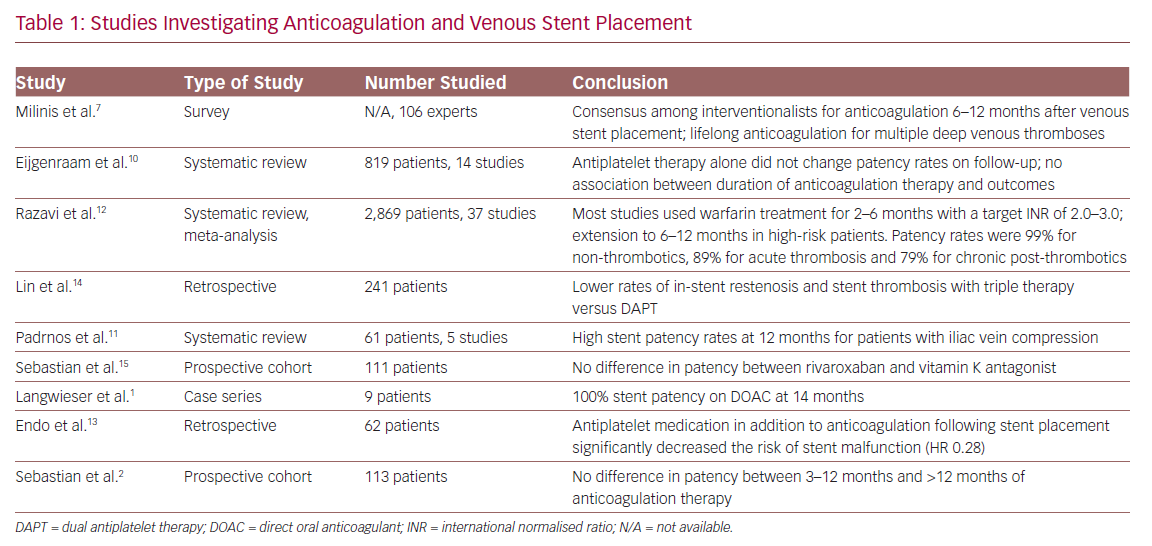
There are no prospective randomised controlled trials demonstrating increased efficacy or superiority of one antithrombotic management strategy over another after the placement of venous stents. The available evidence regarding anticoagulation in this context is limited by heterogeneity in study design, measured outcomes and disparate outcome time points. Furthermore, as the availability of venous-specific stents are a relatively recent development, no long-term data are available regarding technical and clinical outcomes using these devices. Nevertheless, several studies discussing venous stent placement and antithrombotic management are available, which may help guide management. These are summarised in Table 1.
Consensus of Common Anticoagulation Management
At present, there are no consensus guidelines regarding the role of anticoagulation following venous stent placement. Despite this, a recent survey of vascular surgeons, interventional radiologists and haematologists showed a general consensus; anticoagulation is preferred the first 6–12 months post-stent, with lifelong anticoagulation for those with a history of multiple deep venous thromboses. No consensus could be reached on long-term anticoagulation.7
The current body of literature concerning anticoagulation and antiplatelet therapy following stent placement is nearly exclusively in the context of arterial stent placement.8,9 Much of the practice in the realm of venous stents are based off these data. However, as previously discussed, the pathophysiology underlying venous stent stenosis is distinct from arterial stenosis, as there are marked differences in vessel characteristics and flow haemodynamics. Consensus guidelines in coronary stent placement state that triple therapy (warfarin, dual antiplatelet therapy [DAPT]) is recommended (American Heart Association/American College of Cardiology), while in the context of peripheral arterial stent placement, single-agent antiplatelet therapy is recommended (American College of Chest Physicians).
Systematic Reviews and Meta-Analyses
A systematic review conducted by Eijgenraam et al. aimed specifically at assessing anticoagulation following venous stent placement, was unable to demonstrate superiority of any specific antithrombotic agent or regimen.10–12 However, their study had major limitations in methodology; specifically, it combined data from acute and chronic thromboses, and included data in which patients did not receive a stent. A meta-analysis of the data was also not performed.
A meta-analysis by Razavi et al. assessed a total of 37 studies on 2,869 patients who underwent stent placement for iliofemoral venous outflow obstruction;12 however, the number of available studies was inadequate for the comparison of peri-procedural anticoagulation. Post-procedural anticoagulation in the analysed studies commonly entailed warfarin for 2–6 months with a target international normalised ratio of 2.0–3.0. In high-risk patients, anticoagulation was generally extended to 6–12 months. Antiplatelet medication regimens were variable, and lifelong antiplatelet medication was routinely proscribed in some studies. Primary and secondary patency rates with these protocols were 96% and 99% for non-thrombotics, 87% and 89% for acute thrombosis and 79% and 94% for post-thrombotics, respectively. Subgroup analysis was limited by heterogeneity of the abstracted data and underreporting, and no difference was reported between anticoagulation regimens.
A systematic review by Padrnos et al. assessing antithrombotic use in thrombotic May-Thurner syndrome concluded that treatment with anticoagulation for a finite duration of 6 months after stent placement was reasonable, with or without antiplatelet therapy, in the absence of other risk factors. Data from the same study showed that, with the addition of antiplatelet therapy (aspirin and clopidogrel), there were cumulatively improved rates of stent patency and event-free outcomes at 12 months compared with treatment using anticoagulation alone (96% versus 80%).11
Use of Concomitant Anticoagulation/Antiplatelet Therapy
A retrospective study in 2018 examined the effectiveness of anticoagulation alone (warfarin, enoxaparin or a factor Xa inhibitor) versus the concomitant use of aspirin, clopidogrel or DAPT. The study showed higher stent patency (HR 0.28) in patients receiving concomitant antiplatelet and anticoagulation therapy versus anticoagulation therapy alone.13
A second retrospective study assessing triple therapy (anticoagulation with DAPT) versus DAPT alone showed lower rates of in-stent restenosis and stent thrombosis with the addition of anticoagulation, while also maintaining similar levels of major bleeding events.14 This was consistent with a systematic review, which analysed 14 studies on venous stent placement and showed that antiplatelet therapy alone did not change patency rates on follow-up.10
Selection of Anticoagulation and Antiplatelet Agents
For specific anticoagulation agents, warfarin and enoxaparin remain the mainstays for anticoagulation therapy. With the advent of new agents and their integration into other management guidelines, alternate choices of agents have become more frequently utilised. Direct oral anticoagulants (DOACs) in particular have been increasing in use due to their relative ease of use and less need for monitoring. Data on DOAC use following venous stent placement is scant. One small case series of nine patients reported no in-stent restenosis or stent thrombosis at 14 months.1 A recent ongoing prospective cohort study from the Swiss Venous Stent registry compared rivaroxaban or vitamin K antagonists following early stent placement, and showed no significant difference between the two groups in patency or complications.15
Aspirin and clopidogrel are the current preferred antiplatelet agents following venous stent placement, with their use largely extrapolated from experience with arterial stent placement. Newer antiplatelet agents, such as P2Y12 inhibitors, are an interesting prospect, with some studies suggesting lower rates of arterial in-stent restenosis or thrombosis.16,17 While not technically an anticoagulant, cilostazol, a phosphodiesterase-3 inhibitor, has also been shown to affect peripheral arterial angiographic patency.18
Optimal Duration of Anticoagulation Therapy
The optimal duration of anticoagulation therapy following stent placement is also unknown, with few studies addressing this issue. A recent study of 113 patients by Sebastian et al. showed that there was no difference between 3–12 months of post-stent placement anticoagulation and >12 months of anticoagulation, suggesting that discontinuing anticoagulation at 3–12 months is reasonable in this patient population.19
Thrombophilia Testing and Adjustment of Anticoagulation
The role of thrombophilia and thrombophilia testing in the setting of venous stent placement has been inconsistently and incompletely reported in the literature.11 The limited number of studies that have examined stent outcomes in patients with underlying thrombophilia have drawn varied conclusions as to the risk of venous thrombosis. Several studies with under 15 patients have suggested that there may be a higher rate of venous occlusion in those with thrombophilia; however, a single study of 205 patients who underwent iliofemoral venous stent placement noted no difference in patency and re-intervention.20–22
Ongoing Studies
Several large randomised controlled trials assessing outcomes in venous stents are underway, and their results are likely to greatly influence clinical practice. Most relevant to the topic of anticoagulation, especially concerning the need for both antiplatelet and anticoagulation therapy, is the open-label ARIVA (Aspirin® Plus Rivaroxaban Versus Rivaroxaban Alone for the Prevention of Venous Stent Thrombosis in Patients With PTS) trial, whose primary objective is to compare aspirin and rivaroxaban to rivaroxaban alone in patients with endovascular venous stents (NCT04128956). Early studies in a porcine venous stent model demonstrated a reduction in measured platelet deposition in animals that received a direct factor Xa inhibitor compared to those that received antiplatelet agents.23
In the currently enrolling Chronic Venous Thrombosis: Relief with Adjunctive Catheter-directed Therapy (C-TRACT) clinical trial, one of the largest trials investigating endovenous stent placement, patients are placed on anticoagulation and low-dose aspirin (81 mg) for the first 6 months in the absence of contraindications and low-molecular weight heparin at fully therapeutic doses for the first 3 months (Vedantham S, pers. comm., 2018). While C-TRACT focuses primarily on post-thrombotic syndrome, outcomes regarding stent patency and persistent clinical symptom relief are likely to shed light on several important questions regarding perioperative anticoagulation.
Discussion
Currently, there are no large prospective randomised controlled trials that clearly establish a superior efficacy of a particular anticoagulation regimen following endovenous stent placement. Many current management practices are derived from previous literature regarding arterial stent placement, and while some consensus is present among interventionalists, few data are available to inform practice.
In this article, we present several studies that may help inform management. Meta-analyses, systematic reviews and all prospective trials to date have failed to show differences between anticoagulation agents. Most of the available studies suggest the need for postoperative anticoagulation, and a few select, smaller retrospective studies have shown that concomitant antiplatelet therapy may also reduce in-stent stenosis. There are also some early data suggesting the superiority of DOACs for short-term anticoagulation, and given their ease of use compared to vitamin K antagonists, they may be the preferred agent. Finally, multiple studies have concluded that anticoagulation in the 3–12 months after stent placement is likely sufficient in patients without additional risk factors or need for anticoagulation.
Further complicating the issue is that the aetiology of CVD greatly influences stent patency, and therefore, the optimal anticoagulation regimen for each aetiology may be different. In cases of external compression, such as May–Thurner syndrome, where there is relatively maintained vessel wall architecture, data have shown that patency rates are exceedingly high (99%), and therefore anticoagulation may not be necessary.2,12 On the contrary, lower rates of patency in post-thrombotic stents likely relate to the near complete fibrous retraction of the native vessel, whereby the wall is composed nearly entirely of collagen and may also benefit more from one anticoagulation regimen over another. Large enough studies to power subgroup analyses will need to be undertaken to further clarify differences between these patients.
Thrombophilia among patients with venous stents has not been well studied, and the available data do not allow for any scientific conclusions to be drawn on whether thrombophilia should affect antithrombotic management or whether testing for thrombophilia is needed after venous stent placement. At our institution, testing for thrombophilia is left to the discretion of the haematologist, and we do not routinely recommend testing after venous stent placement.
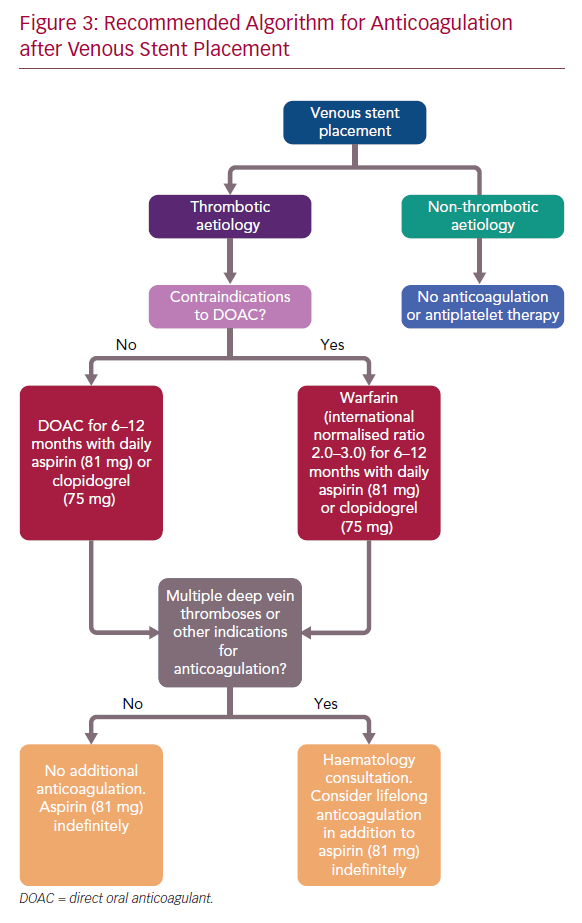
While no consensus guidelines exist, these data suggest the use of anticoagulation in the 3–12 months post-stent placement, along with a single-agent antiplatelet therapy (aspirin 81 mg or clopidogrel), in patients with thrombotic disease without indication for lifelong anticoagulation. At our centre, we prefer the use of a DOAC if no contraindications are present, otherwise warfarin is used (Figure 3). In patients with no history of multiple deep venous thromboses, there appears to be no data to suggest any benefit of extending therapy beyond 1 year. Therefore, we anticoagulate patients for 6–12 months, depending on the quality of venous inflow. Patients with multiple deep venous thromboses, other indications for anticoagulation or additional risk factors are referred for haematology consultation. In these patients, extension of anticoagulation, potentially lifetime, may be reasonable. Additionally, in cases of more complex reconstruction, for example, infra-inguinal stent placement and caval reconstruction, lifelong anticoagulation may also be considered.
In patients presenting with CVD caused by non-thrombotic aetiologies, such as iliac compression syndrome, we do not routinely place patients on anticoagulation, as patency rates are exceedingly high (99%) in this cohort. At this time, data regarding newer anticoagulation and antiplatelet medications are scant, and their use in this setting is experimental.
Several clinical trials involving venous stent placement are underway; their results are eagerly awaited and will likely change the current paradigm. Complicating the issue is that nearly all studies include patients in whom off-label stents were placed. With the advent of several new venous-specific devices and rapid advancements in techniques, ongoing studies will be needed to understand optimal post-procedural management in these patients.
Conclusion
More research is needed to establish the optimal anticoagulation regimen following thrombotic and non-thrombotic venous stent placement, especially with the advent of venous-specific stents. Until these data are available, it is reasonable to place patients undergoing venous stent placement for thrombotic disease on anticoagulation in the 3–12 months post-stent placement, along with concomitant single-agent antiplatelet therapy (low-dose aspirin or clopidogrel). For patients with other indications for anticoagulation, haematology consultation may be considered, and lifetime anticoagulation may be reasonable. Patients presenting with non-thrombotic causes of CVD are likely to not need anticoagulation post-stent placement, as patency rates are exceedingly high in this cohort. At our centre, in the absence of other indications for anticoagulation, we place thrombotic CVD patients on a DOAC and a single antiplatelet agent (clopidogrel followed by low-dose aspirin) for the 6 months following stent placement, with low-dose aspirin to continue indefinitely thereafter.