Peripheral artery disease (PAD) in the lower extremities is the most prevalent cardiovascular disorder worldwide.1 PAD is mainly caused by atherosclerosis; other risk factors include diabetes, chronic kidney disease and smoking.2,3 Endovascular treatment for PAD is constantly being refined and is currently considered an indispensable therapeutic option in addition to exercise and pharmacotherapy. In recent years, treatment with drug-coated balloons (DCBs) has emerged as a novel approach for treating patients with PAD as well as coronary artery disease (CAD). The use of a DCB avoids implantation of a permanent metallic cage and may therefore prevent late complications related to foreign body reaction to the drug, polymer or metal in the vascular wall. Complications from such reactions include in-stent restenosis (ISR), neoatherosclerosis, and late stent thrombosis. Overall, DCBs have resulted in better clinical outcomes compared with conventional balloon angioplasty and bare metal stent implantation.4–6 Another advantage of DCBs is their flexibility. They allow for other options, including treatment for non-stented zones and repeat revascularisation of previously treated segments.
According to the latest European Society of Cardiology guidelines, the use of drug-coated devices is recommended for ISR and short femoropopliteal lesions (i.e. <25 cm), as a Class B treatment option.7 Although drug-eluting stent (DES) technology has improved, the prevalence of both stent fractures (1.9% at 5-year follow-up in some series) and 5-year target-lesion revascularisation (TLR) rate (17%) for treatment of above-the-knee PAD remains high and is inferior to the outcomes reported for CAD treatment.8,9 To overcome these drawbacks, DCB technologies may be a viable treatment option.
In this review, we will describe the current status of both paclitaxel and sirolimus DCB technologies and summarise available clinical trial data for both above- and below-the-knee treatments, as well as discussing safety concerns that have arisen for paclitaxel-based technologies.
Paclitaxel-coated Balloons
Paclitaxel
Paclitaxel is primarily used as an antiproliferative drug for DCBs because of its high-lipophilic characteristics, which allow for passive absorption through the cell membranes and a long-term effect inside the target vessel wall.10 Paclitaxel stabilises polymerised microtubules and prevents their disassembly, thereby suppressing mitotic division, proliferation and migration at the nanomolar level. These effects contribute to preventing neointimal smooth muscle cell overgrowth.
Excipients can also facilitate dissolution of the paclitaxel and its transport into tissues. Without excipients, paclitaxel migration from the DCB into the tissues is limited, as shown in several preclinical studies.11 Despite advancement of coating technology, transportation of paclitaxel into the target vascular tissues is still inefficient, resulting in a loss of at least 30% of loaded paclitaxel into the blood stream during treatment.12 Thus, at present, hydrophilic excipients are indispensable in the paclitaxel balloon for effective drug delivery.11,13
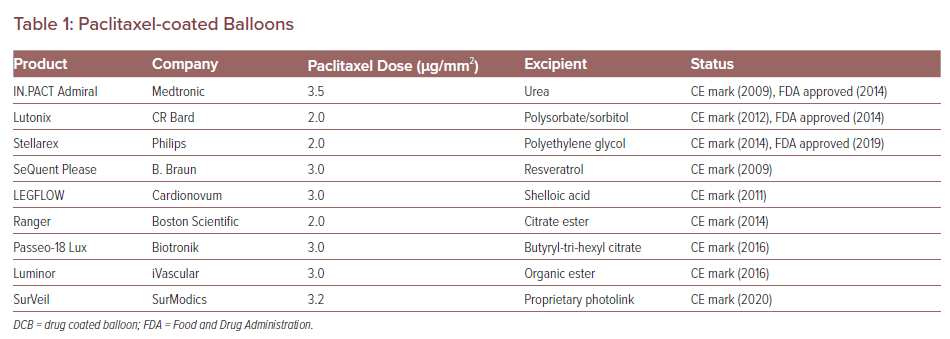
Paclitaxel coating is available in two forms: amorphous and crystalline. An optimal mix of amorphous and crystalline paclitaxel together with the right excipient is necessary for DCB efficacy. The balance of crystalline to amorphous forms affects the pharmacokinetic behaviour of the drug and thus affects neointimal formation and healing. In a preclinical study, DCBs were loaded with the crystalline or amorphous forms of drug in equal doses (3 µg/mm2) and deployed in pigs, after which arterial wall pharmacokinetic levels of the drug were examined.14 Although both formulations achieved similar arterial paclitaxel levels 1 hour after deployment, the crystalline forms retained higher drug concentrations at both 24-hour and 28-day follow-up. So far, more than nine paclitaxel balloons for PAD treatment have received the CE mark and three are approved by the Food and Drug Administration (FDA). The loaded paclitaxel dose and excipient of each device are listed in Table 1.
In experimental models using different doses of paclitaxel delivered from stents, paclitaxel caused dose-dependent increases in tissue necrosis, vascular wall haemorrhage and delayed healing with higher doses.15 Paclitaxel has been used for both drug delivery from DES used in the above-the-knee disease as well as in DCBs as discussed. Nonetheless question about potential toxicities from delivery remain, including the potential for aneurysm formation around paclitaxel-eluting stents. In one single-centre experience of 62 patients treated with the fluoropolymer-cased paclitaxel-eluting stent (Eluvia, Boston Scientific), five aneurysms were identified in the treated segment and thought to be attributable to paclitaxel.16
The IMPERIAL trial was a randomised single-blind non-inferiority study of Eluvia or Zilver PTX (Cook) for the treatment of patients with symptomatic lower limb ischaemia in the superficial femoral or proximal popliteal artery. Retrospective analysis of a subset of patients with duplex ultrasound images found six cases of aneurysm exclusively in the Eluvia group at 1 year.17 Case reports also have been published about the occurrence of aneurysms associated with paclitaxel-coated balloons.18,19 The possible mechanisms of intervention-related aneurysms might be associated with the high dose, high concentration and rapid onset of action of paclitaxel delivered from a drug-coated balloon. This and other safety concerns regarding paclitaxel-coated devices have limited enthusiasm for their use as treatments for vascular disease. We will discuss these safety concerns in the following sections in more detail.
Clinical Studies
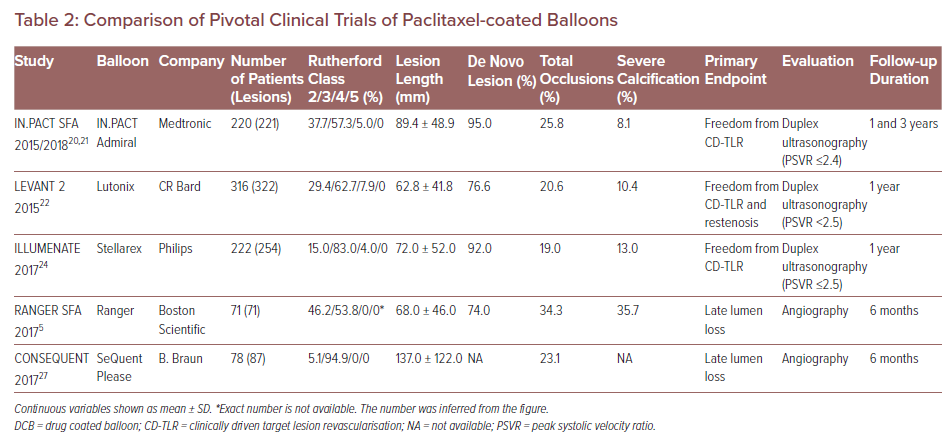
The safety and efficacy of paclitaxel-coated balloons – including IN.PACT Admiral (Medtronic), Lutonix (CR Bard), Ranger (Boston Scientific), Stellarex (Philips) and SeQuent Please (B. Braun) – have been clinically evaluated in randomised controlled trials (RCTs) evaluating them against standard percutaneous transluminal angioplasty (PTA).20–28 However, differences in patient background, definition of endpoints, and follow-up duration among these RCTs make it difficult to differentiate the exact relative performances of DCBs (Table 2). For instance, the study endpoints of some trials were primary patency, defined as freedom from restenosis and clinically driven TLR (CD-TLR) assessed by duplex ultrasound peak systolic velocity ratio (i.e. ≤2.4, <2.5 or ≤2.5, the criteria differ for each RCT) at 1–3 years follow-up.20–22,25
In contrast, in two other RCTs regarding Ranger and SeQuent Please balloon, the primary endpoint was late lumen loss (LLL) assessed by angiography at 6 months.5,27 The studies concluded that paclitaxel DCB was both superior to standard PTA and had a better safety profile for the treatment of patients with PAD. One limitation these RCTs share is the relatively small number of patients enrolled (from 71 to 316). Additionally, although DCB treatment was shown to be superior to standard PTA in terms of study endpoint, DCB treatments need to be compared with each other to determine which device is safest and most effective. Further clinical trials are needed to directly compare the performance of different DCB devices.
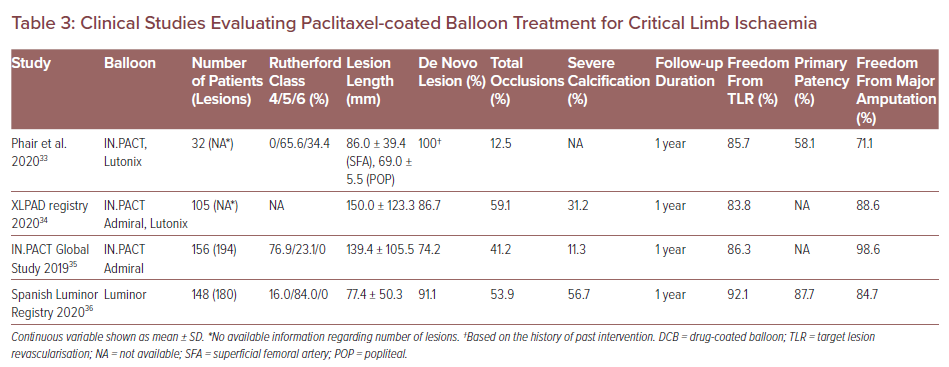
Critical limb ischaemia (CLI) is a clinical end-stage of PAD associated with poor outcomes, with 1- and 5-year mortality rates estimated to be 25% and 50%, respectively.29 Because CLI patients are susceptible to tissue inflammation, anatomical complexity and medical comorbidities, DCB usage can exacerbate their downstream tissue damage due to the risk of distal embolisation in patients with CLI. Several previous clinical case reports and preclinical studies have reported microvasculitis/panniculitis induced by paclitaxel embolisation after DCB treatment and subsequent poor outcomes.30–32 However, to date, the clinical studies evaluating paclitaxel DCB treatment for CLI patients are quite limited (Table 3).
Phair et al. have evaluated the performance of paclitaxel DCB and DES in CLI patients.33 In that study, a total of 88 limbs were revascularised in 88 patients. The DES was used in 56 patients and DCB in 32 patients. Freedom from TLR was not different between patients treated with DES and DCB (90.6% versus 85.7%; p=0.518). However, primary patency and amputation-free survival in the DES group were significantly greater versus the DCB group (80.4% versus 58.1%; p=0.0255 and 88.5% versus 71.1%; p=0.0443, respectively). The authors assumed that distal embolisation of paclitaxel induced by DCB treatment resulted in greater incidence of amputation and mortality compared to DES.
The XLPAD registry demonstrated no significant differences between the DCB and the non-DCB (i.e. stenting and plain balloon angioplasty) group in terms of late outcomes at 1-year follow-up.34 In that registry, a total 327 patients underwent femoropopliteal endovascular intervention (105 DCB versus 222 non-DCB). Although a higher incidence of 12-month major amputation was observed in the DCB group (11% versus 4% in non-DCB; p=0.01), after adjusting for several risk factors the odds of major amputation were not statistically different between the two groups (OR 1.54; 95% CI [0.53–4.51]; p=0.43).
Another two clinical studies suggested that treatment of PAD in CLI patients by DCB is safe and effective with respect to freedom from TLR and amputation-free survival.35,36 In addition, a network meta-analysis demonstrated that paclitaxel DCB has shown encouraging results in terms of primary patency for infrapopliteal lesions in CLI.37 Furthermore, paclitaxel DCB may be better than other treatments (standard PTA and DES) in terms of TLR.
As mentioned above, theoretically paclitaxel DCB has the potential risk of distal embolisation, while the DES has almost no risk of such embolisation. Therefore, although some clinical trials have demonstrated that DCB devices are safe for the treatment of CLI, DES has an advantage regarding the risk of distal embolisation and subsequent worsening of CLI.32,38 Clinicians need to carefully consider the use of DCB in CLI patients. Further clinical trials are needed to understand the risks and benefits in using DCB devices to treat CLI, and improvements in DCB technologies are needed to reduce the risk of distal embolisation.
Safety Concerns Surrounding Paclitaxel Devices
Although several trials have already shown that paclitaxel-coated balloons reduce the rate of restenosis and TLR, a recent meta-analysis has demonstrated increased all-cause mortality at 2 and 4–5 years in patients who underwent paclitaxel-coated device treatment.39 According to this report, all-cause mortality at 1 year after treatment was equivalent between paclitaxel and control devices (i.e. DES and standard PTA) groups. However, the incidence of all-cause death appeared to be significantly greater in the paclitaxel device group after the 1-year follow-up (i.e. at 2 and 5 years). Indeed, there are critical limitations in this meta-analysis that deserve special attention. First, although a significant difference in mortality between paclitaxel and control device groups was evident at 2 and 4–5 years postprocedure, the number of patients involved in the analysis dropped sharply from 1 year (28 RCTs) at both the 2- (12 RCTs) and 5-year (three RCTs) time points. Second, the meta-analysis adopted the intention-to-treat principle without accounting for the rate of crossover to paclitaxel devices. Thus, the relationship between exposure and outcome could be confounded by significant crossover in PTA device of every RCT. Third, the study only demonstrated an association between paclitaxel drug exposure and total death. However, the exact causal mechanisms of death cannot be proven in this study design, which is a critical limitation.
More recently, Rocha-Singh et al. conducted a meta-analysis evaluating the safety of paclitaxel devices, further exploring the increased mortality association.40 A total of 2,185 patients across eight studies with a median follow-up of 4 years were included in this study. As a result, an increased mortality risk associated with the use of paclitaxel devices was observed, with absolute 4.6% increased mortality risk associated with paclitaxel device usage. However, a paclitaxel dose-dependent relationship with mortality was not proved. In long-term observation, loss of follow-up and withdrawal rate in both treatment arms were too high to ignore; inclusion of some of these missing data after further investigation further reduced the mortality risk.
On the other hand, observational studies in 2019 and 2020 demonstrated that the use of paclitaxel devices did not show any correlation with increased mortality.41–44 Secemsky et al. have reported a large Medicare- and Medicaid-based analysis of the relationship between paclitaxel device usage and mortality.41 The authors compared mortality in 5,989 PAD patients treated with paclitaxel devices versus 10,571 PAD patients who received standard PTA (median follow-up duration was 389 days). Multivariate analysis did not show a causal relationship between paclitaxel device usage and mortality (adjusted HR 0.97; 95% CI [0.91–1.04]; p=0.43). In another study, Freisinger et al. evaluated clinical data involving 23,137 patients who underwent endovascular revascularisation.42 A multivariable Cox regression analysis showed paclitaxel DES was not associated with increased long-term mortality for over 11 years follow-up. Moreover, DCB was associated with decreased mortality for the first year past application and not correlated with long-term mortality in the years thereafter.
Recently, a study from Germany has reported the long-term mortality after paclitaxel DCB versus standard PTA for femoropopliteal lesions in real-world clinical setting.43 A total 1,574 patients who underwent DCB (n=1065) or plain old balloon angioplasty (POBA; n=514) treatments were included. Mortality at median follow-up of 51 months was lower in the paclitaxel DCB group (16.9%) than the POBA group (27.8%) (p<0.001). Comorbidities, classic risk factors and disease severity were identified as predictors for death but treatment with a paclitaxel device was not.
The biological nature of paclitaxel is well studied and recognised.45 It is used primarily for chemotherapy at 200 to 400 times the concentration compared to drug levels used in PAD endovascular treatment. Even if frequent DCB ballooning (i.e. more than one long balloon) is performed during one procedure, total paclitaxel doses cannot reach nearly the levels seen during systemic chemotherapy. Moreover, plasma pharmacokinetic levels of paclitaxel do not reach the level causing adverse effects as reported in cancer treatment. How a crystalline drug (i.e. paclitaxel) even if it embolized to non-target organs, such as the lungs, could be linked to a patient's death years later still needs to be elucidated.
The FDA reported a provisional warning on continuing DCB use in January 2019.46 Two updates were issued in March and August 2019, the latter after a public meeting of the Circulatory System Devices Panel. The FDA has stated that further observation is required and there is still no clear evidence of the mechanism by which paclitaxel could cause mortality.47 “For many patients, alternative treatment options to paclitaxel DCB and paclitaxel DES provide a more favourable benefit–risk profile based on currently available information. For individual patients judged to be at particularly high risk for restenosis and repeat femoropopliteal interventions, clinicians may determine that the benefits of using a paclitaxel-coated device outweigh the risk of the late mortality".48
Sirolimus-coated Balloons
Sirolimus
Sirolimus, also known as rapamycin, is a macrolide compound that was initially discovered as an immunosuppressive agent. In the 1990s, seminal studies revealed the agent as a potent inhibitor of smooth muscle cell proliferation and migration, and only then did the potential of rapamycin for cardiovascular therapeutics emerge. Sirolimus inhibits mammalian target of rapamycin complex that promotes the translation of cyclin D1 mRNA, one of the cell cycle regulators.49 In animal studies, as discussed above, localised areas of inflammation and cell toxicity are observed for paclitaxel-containing devices. However, sirolimus is a cytostatic agent with the ability to inhibit cell division without creating vascular toxicity.50 In one animal study, no differences in histological endpoints, such as endothelialisation, were found with low- (64 µg/stent) or high-dose (196 µg/stent) sirolimus-eluting stents in the rabbit iliac model although neointimal suppression was better for the latter.51
Despite the beneficial therapeutic safety margin and anti-restenotic effects that have led to sirolimus becoming the preferred coating for coronary artery intervention, sirolimus and other -limus drugs present a challenge when used on balloons.52 Because tissue bioavailability – which affects both drug uptake and retention – is lower for sirolimus than paclitaxel, absorption enhancers are required to improve tissue uptake. A previous preclinical study showed that a balloon with novel phospholipid-encapsulated sirolimus nanocarrier coating achieved efficient transfer of sirolimus to all layers of the vessel wall, with a high tissue concentration persisting for days after application.53 Moreover, recently reported clinical and preclinical studies have shown the efficiency and safety of sirolimus DCB treatment for the coronary artery.54,55 In conjunction with the safety concerns for paclitaxel mentioned above, there is a growing movement to develop effective sirolimus DCBs.
Clinical Studies
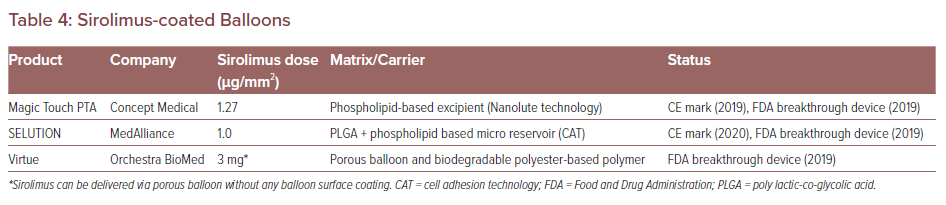
To date, there are three commercially available sirolimus DCB devices. These are Magic Touch PTA (Concept Medical), SELUTION (Med Alliance) and Virtue (Orchestra BioMed). The characteristics of each sirolimus DCB are listed in Table 4. The clinical trials of these devices will be conducted in the US. Magic Touch PTA, SELUTION and Virtue have been granted FDA breakthrough device designation.56–58
The novel sirolimus DCB has been tested in PAD treatment. The first-in-man XTOSI study is a clinical registry to investigate the safety and efficacy of Magic Touch PTA in the treatment of femoropopliteal and below-the-knee arterial lesions.59,60 The study endpoint was primary patency at 6 months determined by a duplex ultrasonography (criteria of restenosis: peak systolic velocity ratio ≤2.4). This registry includes 33 patients with relatively severe disease characteristics, i.e. >90% of patients had CLI (Rutherford category 5 or 6), and approximately 80% of patients had at least one total occluded lesion in below-the-knee arteries before angioplasty. Technical success rate was 100% in the initial treatment procedure. In this study, limb salvage was achieved in 975 of patients at 30 days. Primary patency and freedom from CD-TLR at 6 months were 82% and 91%, respectively. There was no evident distal embolisation or slow flow phenomena after application of sirolimus DCB in the below-the-knee lesions. Freedom from device- and procedure-related mortality was 100%.
The results of the SELUTION first-in-human study, assessing the safety and efficacy in treatment of superficial femoral artery and popliteal artery, were also recently reported.50 The Cell Adherent Technology increases drug uptake into the arterial wall, prolongs exposure to the sirolimus, reduces dose loss to circulation, and minimises embolisation. In this trial, 50 patients with complex superficial femoral artery disease (30% total occlusions, 34% moderate or severe calcification, and target lesion length 64.3 mm) were treated with the SELUTION DCB. The primary endpoint of this study was angiographic LLL at 6 months. The study demonstrated that SELUTION achieved its 6-month LLL. The mean LLL was 0.29 ± 0.84 mm, which was significantly lower than the 1.04 mm objective performance criterion value for uncoated balloon angioplasty (p<0.001). Moreover, freedom from angiographic binary restenosis and duplex ultrasound primary patency at 6 months were 91.2% and 88.4%, respectively. The clinical improvement in Rutherford classification, ankle–brachial index, walking impairment, and quality-of-life at 6 months were improved and were significantly improved from 6 to 12 months follow-up (p<0.001, respectively). The 2-year results of the SELUTION study are currently under analysis. These findings will likely confirm the efficacy of sirolimus-coated balloon technology.
In CLI treatment, the PRESTIGE below-the-knee clinical trial is on-going (NCT04071782). The objective of this trial is to evaluate the 6-month safety and performance outcomes of SELUTION for the treatment of long tibial occlusive lesions in patients with CLI. In total, 22 patients have now been enrolled and clinical follow-up will be at 1, 3, 6 and 12 months.
Sirolimus DCBs will offer a new approach to the endovascular treatment for PAD. Regarding coronary artery interventions, several trials have already shown the safety and efficacy for sirolimus DCB.61–63 A Nanolutè study (Magic Touch) has evaluated the long-term efficacy of sirolimus DCB for CAD. This study involved 408 patients (the sirolimus DCB was used for 183 patients with ISR, 185 with de novo small vessel lesion, and 40 with de novo large vessel lesion). Overall, the rate of major adverse cardiovascular events at 24 months was 4.2% (three cardiac deaths, 13 TLR, and one target vessel MI). The authors concluded that sirolimus DCB is a safe and feasible option for patients with ISR and for those with de novo lesions. Currently, the data obtained from preclinical and clinical studies are not enough to conclude the safety and efficacy of sirolimus DCBs and further studies are required to establish endovascular treatment using this approach.
Conclusion
DCBs have emerged as a newer treatment option for obstructive PAD and CAD and may offer some advantages compared with DES. Paclitaxel DCBs for the treatment of PAD have shown efficacy over plain PTA in several RCTs. However, a recent meta-analysis suggests an association between increased mortality and paclitaxel device usage, although a causal relationship is still being discussed. Alternatively, sirolimus DCB devices currently in development are attracting attention in the field of PAD and CAD treatment. Although it may be too early to conclude the safety and efficacy of sirolimus DCB over paclitaxel DCB, future DCB technologies will continue to improve and offer more promising treatment options with improved efficacy and safety.