Deep venous thrombosis (DVT) affects approximately one in 1,000 patients yearly, and 40–70% of these patients will develop post-thrombotic syndrome (PTS) in their lifetime: a constellation of symptoms and signs of chronic venous insufficiency, including pain, swelling, varicose veins, and ulcerations.1,2 PTS is associated with profound morbidity and cost, which justifies the attention it has received in recent years in the form of randomised controlled trials (RCTs) on how to decrease its incidence.3–5
The pathophysiology of PTS is believed to be a culmination of outflow obstruction, valvular damage leading to reflux, and chronic inflammation secondary to thrombosis.6 The ‘open vein hypothesis’ endorses the relieving of the venous obstruction in order to improve flow and decrease the risk of reflux, thereby reducing the chance of developing PTS.7 Anticoagulation at this time remains the mainstay of treatment for DVT, with dose durations varying based on aetiology according to the American College of Chest Physicians (CHEST) guidelines.8 Prolonged anticoagulation, however, has not been shown to reduce the risk of PTS, and recanalisation of the iliac vein is rare after a DVT.9
Some view the results of the Extended Anticoagulation Treatment versus Standard Treatment for the Prevention of Recurrent Venous Thromboembolism (VTE) and Post-thrombotic Syndrome in Patients Being Treated for a First Episode of Unprovoked VTE (ExACT) study as evidence supporting the open vein hypothesis. That study was an RCT comparing standard and extended regimens of anticoagulation that demonstrated no differences in the risk of PTS or in quality of life (QOL).9
The landmark Catheter-directed Venous Thrombolysis (CaVenT) and Acute Venous Thrombosis: Thrombus Removal with Adjunctive Catheter-directed Thrombolysis (ATTRACT) trials were the first to show a benefit with catheter-based interventions in preventing or alleviating PTS in patients with first-time DVT. The former found an absolute risk reduction in the development of PTS of 14.4% at 2 years and 28% at 5 years, while the latter demonstrated lower incidence of moderate (Villalta score >9) and severe (Villalta score >15) PTS, faster pain relief, and improved QOL for patients with iliofemoral DVT.3,4 The most recent Catheter-directed Thrombolysis versus Anticoagulation (CAVA) trial, which randomised patients with iliofemoral DVT to ultrasound-assisted thrombolysis or anticoagulation, failed to show a benefit in preventing PTS development at 1 year follow-up.5 The conflicting results of the studies have raised criticism mainly with regard to incorrect patient inclusion or technical inappropriateness.10
We need to acknowledge, otherwise, that catheter-based interventions come at a cost. Although no difference in mortality has been shown, when compared with anticoagulation alone, catheter-directed thrombolysis (CDT) has been associated with higher rates of blood transfusion, pulmonary embolism, intracranial haemorrhage, and vena cava filter placement. In some countries, CDT is also associated with longer hospital stay and threefold higher hospital costs.11 Despite these disadvantages, current evidence helps us to better select our patients, experience keeps improving, and the popularity of endovenous treatment will continue to grow. With the advent of new devices, it is imperative for vascular surgeons and interventionalists to increase their familiarity with the techniques and to choose their patients wisely.
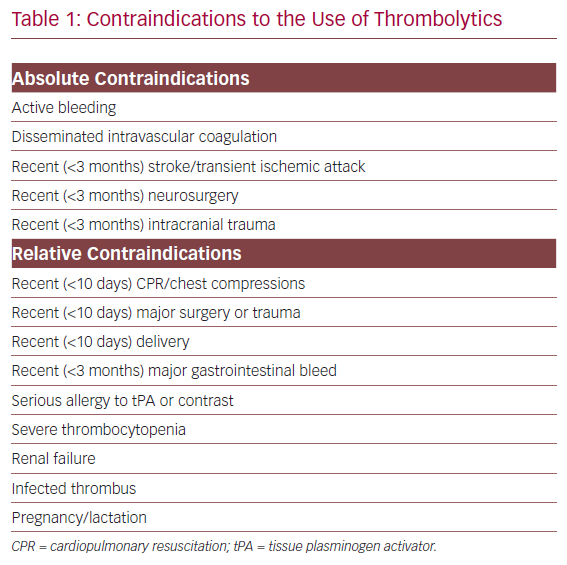
Patient Selection Algorithm
The ATTRACT trial demonstrated no benefit of intervention for patients with femoropopliteal DVT.12 The CaVenT study demonstrated successful outcomes in patients with up to 21 days of symptoms, but we have noted an approximately 90% technical success rate in patients with symptoms for up to 30 days at our institution.3,13,14 It is thus our practice to consider intervention for patients with iliofemoral DVT and symptoms for less than 30 days.
Symptom severity represents the next decision point with regard to who and how to intervene. For patients presenting with limb-threatening ischaemia (phlegmasia), emergency clot removal is self-explanatory as the only option, while asymptomatic patients would not qualify for any intervention.15 The difficulty in decision-making is for those with mild–moderate pain and swelling. Patients with moderately severe symptoms are typically monitored on IV heparin infusion for 24–48 hours. If symptoms persist or worsen even with ambulation, intervention can be offered, provided the patient is ambulatory with acceptable life expectancy. A small subset of this group may opt for immediate clot removal (e.g. young, athletic patients) after a thorough discussion of expectations and risks.
Among those who warrant intervention, bleeding risk is the final criterion that must be evaluated. Pharmacomechanical thrombolysis and/or catheter dripping of thrombolytics are generally reserved for patients with low bleeding risk. High bleeding risk patients (Table 1) should be treated with aspiration thrombectomy with minimal or no use of thrombolytics.16 Those with moderate bleeding risk are treated at the surgeon’s discretion with pharmacomechanical thrombectomy (PMT) and/or aspiration thrombectomy with sparing use of tissue plasminogen activator (tPA; Figure 1).
Optimising Outcomes
Achieving optimal results requires far more than selecting the right patient and technique. A certain to do list needs to be followed to minimise failure. Herein we summarise our institution’s recommendations to prolong patency.
Thrombus Clearance
Achieving thrombus clearance of >90% is of paramount importance. Mewissen et al. found in their review of 221 iliofemoral DVTs within the national venous registry a clinical success (>50% thrombus clearance) rate of 83%. Primary patency was significantly better in patients with >50% clot removal (85%) compared with those with significant residual disease (36%).14 In our investigation of 142 patients who underwent CDT/PMT and stenting, technical success was achieved in more than 90% of patients with a 1-year primary stent patency of 83.1%. The strongest predictor of stent thrombosis was incomplete lysis (<50% thrombus clearance), HR 7.41. Furthermore, incomplete lysis was also the strongest predictor of the development of PTS in 5 years.17
Stenting
In the landmark trials, stenting of identifiable obstructive lesions after DVT lysis was performed in less than 50% of cases.3,4,14 However, stenting has evolved to become an essential component of acute DVT intervention given that multiple studies have demonstrated improved patency with adjunctive stenting after thrombolysis. Mewissen et al. demonstrated 1-year primary patency of 74% with stenting compared with 53% without.14 Similarly, Meng et al. from China randomised 74 patients with >50% venous obstructive lesions discovered after thrombolysis to stenting versus no stent placement. The stented group benefitted from significantly greater primary patency rates (86% versus 55%) and improvements in symptomatology based on various clinical scales.18 Current guidelines recommend the use of self-expanding stents in iliocaval lesions that are uncovered by thrombus removal.15 Current convention defines 50% stenosis as the threshold for stenting. The Venogram Versus Intravascular Ultrasound for Diagnosing and Treating Iliofemoral Vein Obstruction (VIDIO) trial validated this threshold and reported 6-month clinical improvement with stenting of thrombotic lesions causing >54% area reduction.19 In a 2015 meta-analysis, 1-year stent primary patency was 87% for acute thromboses.20 Stenting rates have been steadily increasing since the early trials and continue to do so with the advent of novel dedicated venous stents. In the same meta-analysis, stenting in acute DVT ranged from 34% to 100%.20
In principle, optimal outcomes are achieved when the stent lands in healthy vein segments proximally and distally. Recent studies (including from our institution) have reported mixed results regarding duration of lysis treatment and stented vein length.21–23 In our experience, we found no difference in stented length between single PMT sessions and staged CDT/PMT interventions.21 Historically, longer stents have been associated with inferior patency, but no differences were found in these studies.21–24 Regarding extension to the infrainguinal level, there is little controversy regarding its necessity when disease extends to that level. However, patency and long-term outcomes will likely be inferior, mainly due to the extent of the disease and not because of stent complications (fractures are very rare). Neglen et al. demonstrated an association with worse secondary patency, with infrainguinal extension of stents mirroring the severity of the chronic disease.25 In our institution’s experience, stenting into the common femoral vein was not predictive of stent failure, at least, for our 3-year follow-up. It was predictive, however, of PTS development, which is also a sign of more extensive disease.21
Proximal extension of the stent into the vena cava has been recommended to guarantee complete coverage of the proximal iliac vein lesion/compression and to compensate for the reduced peripheral radial forces of the traditionally used Wallstent (Boston Scientific). Stent extension into the vena cava, however, jails the contralateral iliac vein, and there has been increasing concern regarding development of contralateral DVT. According to our own experience, we found no significant association between caval extension and contralateral DVT development, as recurrent DVT seems to occur irrespective of stent placement; still, we cannot ignore it is a precipitating factor.17 Khairy et al. published a 4% contralateral DVT rate for 376 patients (84% Wallstent) at two institutions, while a 2019 systematic review by Duarte-Gamas et al. reported an incidence ranging from 0 to 15.6% in 1,864 patients.26,27 Novel stents with their uniform radial force have reduced the need to extend far into the vena cava.
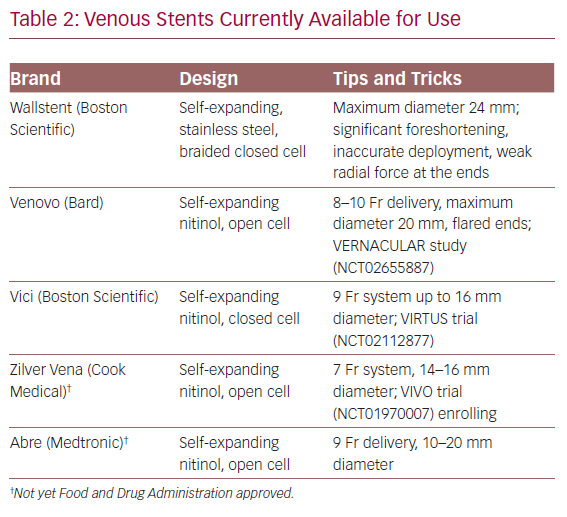
Dedicated venous stents have been available over the past few years in Europe and have recently been Food and Drug Administration approved and entered the US market (Table 2). Gone are the days when we resorted to using stents designed for the smaller and more dynamic arterial system. Arteries bear a different haemodynamic load compared to larger, low flow, externally compressed, or scarred veins. Large venous stents provide better inflow and outflow avenues. Raju et al. investigated the utility of iliac vein stent oversizing by 2 mm compared to the anatomic norms and confirmed larger flow channels on intravascular ultrasound (IVUS) and better clinical outcomes.28 It is thus our practice to use 16–18 mm stents in the common iliac vein, 14 mm stents in the external iliac vein, and 12–14 mm when extending distally to the common femoral vein.
Intravascular Ultrasound
The utility of IVUS (Philips) cannot be overstated. In contemporary practice, IVUS is essential to identify obstructive venous lesions, guide stent diameters, landing zones and to confirm a satisfactory final outcome. The 2017 VIDIO trial compared measurements of venous anatomy in 100 patients using both multiplanar venography and IVUS.29 Use of IVUS led to the detection of clinically significant vein stenosis (>50% stenosis) in 81 patients versus 51 patients via venography. More importantly, the use of IVUS led to a change in treatment plan in 57 patients: 54 patients received stents for lesions not otherwise seen on venography and 3 patients had false positive findings on venography that were not seen on IVUS.29 Authors of the VIDIO trial concluded that IVUS was both more sensitive and more specific than venography for identifying and sizing iliac vein stenosis.29 Furthermore, a subsequent publication reported that clinical improvement could be predicted by the percent change in vein diameter before and after treatment.19 However, at this time, long-term patency with and without the use of IVUS has yet to be compared.
Catheter Techniques
Here we aim to describe the main thrombectomy techniques/devices in the deep venous armamentarium.
Catheter-directed Thrombolysis
CDT or lytic dripping is the standard baseline technique for delivering tPA into the thrombus, softening it. It can be achieved through a 5 Fr system with femoral or popliteal vein access. A multi-sidehole infusion catheter can be manoeuvred over a wire until it is embedded in the iliocaval thrombus. Through this catheter, a continuous infusion of 1 mg/h tPA is delivered. Simultaneously, 500 units/h of unfractionated heparin is infused through the sheath sideport. The patient’s neurologic function is evaluated every 2 hours, usually in the intensive care unit. Complete blood counts and coagulation levels (e.g. fibrinogen) are checked every 4–6 hours. The patient is brought back to the angiography suite for lysis check versus termination and stenting every 8–24 hours. Performed alone, this technique may take 24–48 hours to complete. Technical success rates with CDT alone range from 83% to 100%.3,4,12,24 Bleeding complications ranged from 4% to 9% which included haematomas or transfusion requirements.3,4,17 A 2016 Cochrane review, the CaVenT study and ATTRACT trial reported no incidences of stroke or intracerebral haemorrhage.3,4,30 Overall, in the right patient, CDT is effective and safe. However, due to the prolonged tPA infusion times and increased costs, the authors’ practice has gradually shifted to a single-stage, PMT-only approach with adjunctive CDT if thrombus clearance is unsatisfactory.
Ultrasound-assisted CDT
Standard dripping can be enhanced with ultrasound, which has shown increased clot lysis in vitro.31 This is the theory behind the EkoSonic catheter (EKOS). The EKOS catheter is the multi-sidehole infusion catheter combined with a high-frequency ultrasound transducer to increase lytic penetration into the thrombus. The technique for ultrasound-assisted (UA) CDT is similar to standard dripping except for the need for normal saline coolant and the ultrasound tower. Patients are similarly monitored for neurologic changes and abrupt changes in their laboratory work. The recently published RCT comparing UACDT versus anticoagulation (CAVA trial) showed no difference in PTS development, PTS severity, or QOL at 1 year.5
Currently only one RCT has compared standard CDT and UACDT: the Ultrasound-enhanced Thrombolysis versus Standard Catheter-directed Thrombolysis for Iliofemoral Deep Vein Thrombosis (BERNUTIFUL) trial.32 Engelberger et al. randomised 48 patients to 20 mg tPA over 15 hours via UACDT versus CDT. At the end of 15 hours, both groups had significantly less thrombus burden compared with baseline but there were no significant differences seen between groups in clot clearance, hospital length of stay, or 3-month patency. No differences were found at 1 year as well, and the authors concluded that the addition of ultrasound energy to CDT had no impact on clinical outcomes.32,33
Rheolytic Thrombolysis
In line with the Society for Vascular Surgery/American Venous Forum guidelines for early thrombus removal,15 our own institution’s practice is a ‘PMT-first’ approach followed by selective CDT with the goal of completing clot removal in one session. This is performed most often with the AngioJet catheter (Boston Scientific). The AngioJet catheter works in two phases. The first is the power-pulse mode in which 6–10 mg tPA in saline solution is forcefully sprayed into the thrombus. After 30 minutes to allow tPA to soften and partially dissolve the clot, the thrombectomy mode is activated. Using multiple directional saline jets, a pressure gradient is created that draws softened thrombus into the multiple inflow windows of the catheter and then into the collection bag. In 2015, Garcia et al. published the results of the Peripheral use of AngioJet Rheolytic Thrombectomy with a Variety of Catheter Lengths (PEARL) study, which describes outcomes after peripheral use of AngioJet thrombectomy in DVTs. Twelve-month patency was 83% with a 3.6% bleeding event rate, leading the authors to conclude that rheolytic PMT is a safe and effective potential alternative to CDT.34 The Zelante catheter (Boston Scientific) is the latest development of the AngioJet technology. This 8-Fr system utilises a singular, larger power-pulse, saline jet, and single inflow window, which allows the operator to control and focus on a specific area by rotating the catheter in order to effectively treat the vessel wall circumferentially.
Clinical guidelines recommend a PMT-first approach over CDT based on similar efficacy and potentially better safety.15 Two meta-analyses from China demonstrated that PMT ± CDT is not inferior and may be superior to CDT alone.33,34 One study reported no difference in Villalta scores while the other stated that PMT achieves lower scores. Both studies concluded shorter hospital stays, lysis time and volume, with similar bleeding complication rates.35,36 One particular study by Lin et al. compared CDT (n=46) and AngioJet (n=52) and found no differences in technical success and symptom relief.37 The PMT group, however, underwent significantly fewer venograms, less lytic infusion time (76 minutes versus 18 hours), and shorter ICU stays (0.6 versus 2.4 days). Safety profiles were similar but with the CDT group requiring more blood transfusions.37 In our experience, AngioJet PMT in a single session was not associated with any significant differences in technical success, differences in stented length, or long-term patency.21 PMT alone was also associated with fewer trips to the operating room and no difference in clinical improvement when compared with CDT.13 Rheolytic PMT, however, is not infallible. AngioJet use is associated with acute kidney injury (AKI) in up to 20% of patients.38,39 In our own experience, >95% of these AKIs are transient and resolve in the first 30 days after the procedure, and can be eliminated by staging extensive iliocaval DVTs (starting with a few-hour lytic drip), thus lowering the thrombectomy volumes.40
Aspiration
Aspiration techniques have been traditionally reserved for patients who would benefit from early thrombus removal but have a contraindication to receiving pharmacological thrombolysis. More recently, novel aspiration thrombectomy devices have entered the market and increased utilisation is seen, justified by the known risks and time-consuming nature of thrombolytics. Safety and efficacy against thrombolytics/rheolysis and PTS development have yet to be described.
Syringe Aspiration
Aspiration using a large-bore catheter and syringe is the simplest possible technique and can be selectively performed when small amounts of clot are present. A Turkish RCT compared a 9 Fr catheter/20 ml syringe aspiration system to anticoagulation. Technical success (unobstructed venous flow) was achieved in 90.4% of patients (n=19). At 1-, 3-, and 12-month follow-up, the aspiration group had significant improvement in clinical symptom scores. Although significantly higher than the anticoagulation control group, 1-year primary patency was only 57.1%.41
Penumbra Indigo
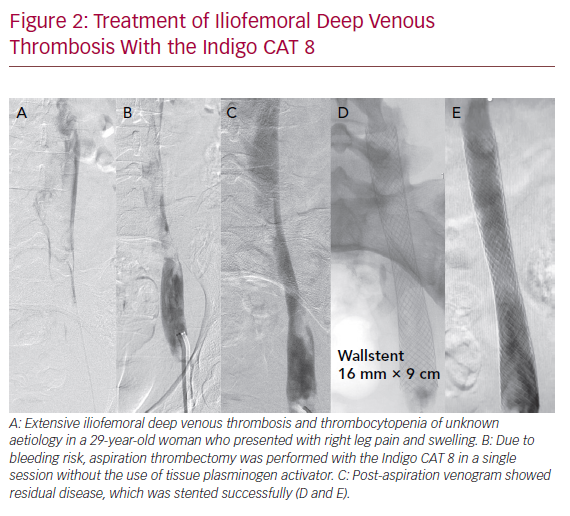
The Indigo CAT 8 (Penumbra) is a single-use, powerful 8 Fr aspiration catheter that can aspirate up to 160 ml/s. It consists of three components: a suction catheter, a separator for fragmentation and cleaning, and the vacuum pump. Currently, no studies exist comparing the Indigo CAT with pharmacological techniques. One retrospective series of 10 patients had >70% thrombus clearance in a single session in six patients, while the remaining four required adjunctive percutaneous methods of clot removal. Long-term patency data were not available. However, no bleeding complications were reported, and none of the patients required transfusion.42 Indigo CAT 8 may provide an alternative to thrombolysis infusion or PMT (Figure 2).
AngioVac
The AngioVac (AngioDynamics) is a large veno-venous filtration system requiring drainage and reinfusion cannulas. The suction catheter requires 26 Fr venous access, one of the device’s limitations. Due to its large size, it is most frequently used for caval and iliac thromboses. Limited data on the AngioVac system exist. A meta-analysis found that patients undergoing AngioVac aspiration for thrombotic indications had successful recanalisation rates of approximately 80%.43
Inari FlowTriever and ClotTriever
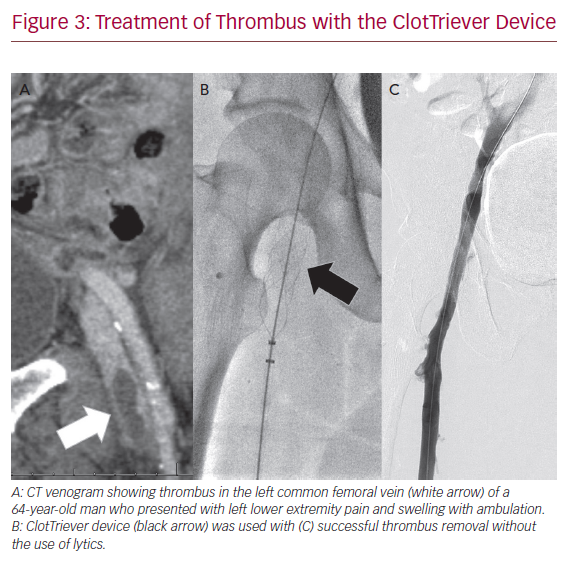
The FlowTriever and ClotTriever devices (Inari Medical) combine both aspiration and mechanical thrombectomy into one device without the need for pharmacological lysis. They are currently available in the US only. The FlowTriever consists of a 16–24 Fr aspiration sheath that is advanced up to the distal end of the thrombus, which is then drawn to the vacuum sheath. Thrombectomy can be assisted as needed with nitinol discs that engage the clot and pull into the FlowTriever sheath. The ClotTriever is a 13 or 16 Fr, mechanical thrombectomy system with a catheter coring element and collection bag that is deployed proximal to the thrombus and retracted into the sheath. Successful use of these devices is available as case reports and small series (Figure 3). The most recent series of 12 patients demonstrated 100% technical success, 92% clinical improvement, and 80% patency at early follow-up.44
Conclusion
Multiple RCTs have found symptomatic benefit of early percutaneous DVT debulking in accordance with the open vein hypothesis. Multiple academic and clinical venous societies have incorporated percutaneous treatment recommendations into clinical guidelines for the treatment of DVT. Although anticoagulation and compression remain the mainstay of treatment, patients with moderate swelling and pain, low bleeding risk, and good life expectancy could potentially be treated with a combination of pharmacological and mechanical thrombectomy methods. These procedures are generally safe but do confer an increased risk of bleeding or AKI. Thus, patient and technique selection should be of utmost importance. Regardless of the treatment modality, physicians should strive for complete clot clearance, and residual disease should be assessed on IVUS and subsequently stented. With the increasing popularity of percutaneous thrombus removal, it is essential to familiarise oneself with the who, when, and how of venous thrombosis treatment to provide effective and durable symptom relief to our patients.