History and Challenges of Abdominal Aortic Aneurysm Suppression Research
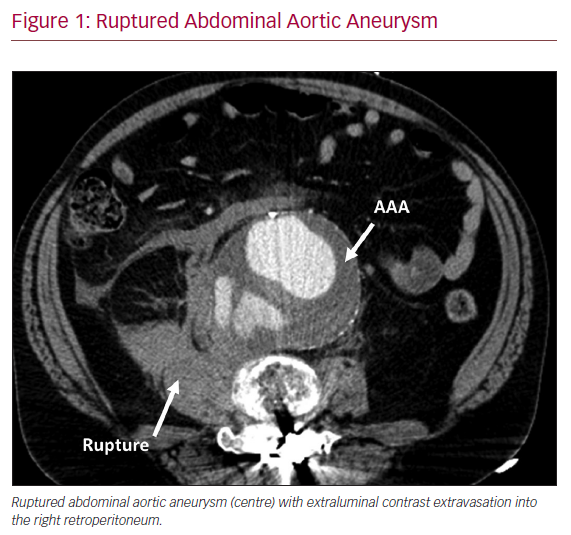
Abdominal aortic aneurysm (AAA) is a common and lethal disease in the US, affecting more than 1 million men and women over 50 years old.1 The natural history, if left untreated, is one of progressive aneurysm enlargement, rupture and sudden death (Figure 1). Current management guidelines call for surgical repair of aneurysms ≥5.5 cm in diameter in men or ≥5.0 cm in women, based on evidence that population screening reduces AAA-related mortality by >40%.2
In the US, at-risk Medicare beneficiaries ≥65 years old undergo ultrasound screening for AAA disease. More than 90% of AAAs identified at screening, or as an incidental finding on cross-sectional abdominal imaging studies ordered for other reasons, are below the size thresholds recommended for surgical repair.1 Thus, most affected individuals are entered into surveillance programmes at the time of diagnosis to monitor disease progression, with 70% or more ultimately requiring surgery at a later time point.2
AAA Disease Progression can be Closely Monitored
When excluding aortic conditions such as Marfan or Ehlers–Danlos syndromes, or mycotic or traumatic aortic aneurysms, the remainder of infrarenal aortic aneurysms are considered ‘atherosclerotic’, in that they share many of the same risk factors as the broader category of cardiovascular diseases (CVD). These atherosclerotic aneurysms are typically asymptomatic until the time of impending rupture, and typically enlarge at a predictable rate of 2–3 mm/year, depending on baseline diameter and associated risk factors.3 Importantly, current smoking increases the rate of enlargement by 35% compared with non-smokers. Although the ultimate goal of AAA suppression is to prevent rupture and sudden death, larger aneurysms are surgically repaired and thus censored from further follow-up. Given that the aortic diameter/rupture risk relationship is reasonably well-established, maximum diameter is the primary clinical marker used to monitor disease progression.4
AAA Pathobiology is Coming into Focus
Key pathological features associated with aneurysm enlargement include progressive medial elastin and smooth muscle cell depletion, mural leucocyte accumulation and angiogenesis, and laminar accumulation of luminal thrombus. Infiltrative mural leucocytes, including monocytes/macrophages, neutrophils, mast cells, B-cells, and CD4 and CD8 T-cells promote aneurysmal aortic degeneration via production of extracellular matrix-degrading metalloproteinases and other proteases, pro-inflammatory cytokines and lipid mediators, angiogenic factors, and reactive oxygen species (ROS).5–14 We and others have demonstrated that interventions effective in limiting aortic macrophage accumulation, including hyperglycaemia and exercise-induced aortic hyperaemia, as well as apelin, rapamycin, angiotensin II type 1 receptor blockers, inhibition of CXCL4-CCL5 dimerisation and hypoxia inducible factor inhibition, are particularly effective in suppressing experimental aneurysm progression, underscoring the significance of aortic mural inflammation in aneurysm pathobiology.15–22
Limited Translation of Research Advances Into Effective Clinical Therapies
Despite apparent progress in understanding the mechanisms fundamental to AAA pathobiology, as outlined above, and the fact that most AAAs are identified when they are small, allowing for years of surveillance and potential pharmacological intervention, no class of medication, including statins, angiotensin-converting enzyme inhibitors (ACEIs) or receptor blockers, beta-blockers, anti-proteolytic, anti-inflammatory, anti-angiogenic, or immune modulating agents, has proven effective in limiting clinical AAA enlargement.4,23 The absence of an effective inhibitory strategy for early AAA disease greatly increases the likelihood that patients will ultimately need surgery, regardless of their overall health, advanced age, or comorbid conditions, with substantial attendant mortality and morbidity.4 Knowing that an AAA is present, but still below the threshold required for surgical repair, leads to significant decrements in the quality of life of ‘worried well’ patients at risk for disease progression and rupture.24,25
Need for Identification of Safe and Effective Alternatives to Surgical Repair
Significant societal benefits will accrue from identifying an inexpensive, relatively non-toxic and easy to administer pharmacological agent effective in suppressing early AAA disease. More than 20 years ago, the National Heart, Lung and Blood Institute identified medical management of AAA disease as a significant unmet medical need, with substantial financial and intellectual support for this critical research area continuing since that time.26 The Society for Vascular Surgery, ranking the top 50 research priorities to improve health for patients with vascular disease, placed identification of a safe and effective medical agent to limit progression of AAA disease near the top of a list that included interventions to limit amputations, prevent strokes and improve the quality of life for patients requiring haemodialysis, among others.27
Despite progress in understanding aneurysm biology, and some reduction in AAA-related mortality in the past decade,28 AAA remains a leading cause of death in adult Americans and is still without effective therapeutic options short of major surgery. Many pharmacological strategies have been trialled, albeit none successfully to date (Table 1).23,29–31 Even in retrospective analyses, no agent otherwise known to be effective in the prevention of cardiovascular (CV)-related endpoints has been linked to reduced prevalence or progression of AAA disease.32
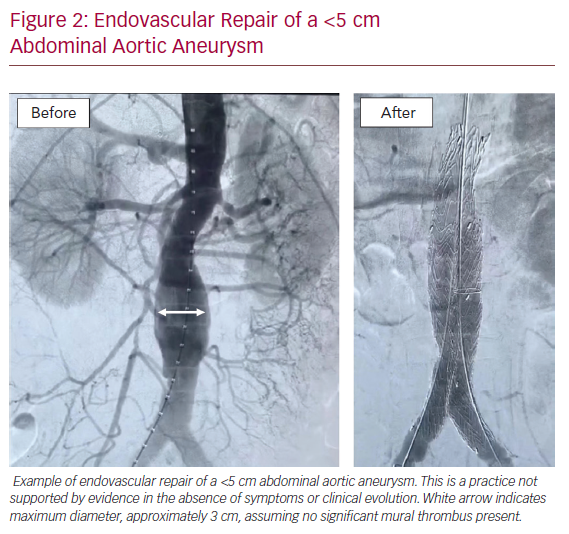
In the absence of effective medical therapies, care today often defaults to an excessive reliance on surgical intervention for small AAAs, a practice not supported by evidence.33 These interventions cost an estimated >US$1m for every AAA-related death prevented by surgical intervention.34 Two level I trials have clearly demonstrated that surgery for AAA <5.5 cm in diameter, even when performed percutaneously with endoluminal grafts, is not justified based on safety or cost considerations (Figure 2).35 However, no other effective treatment options have been identified, other than cessation of cigarette smoking for those who are still smoking. Thus, there is a compelling societal benefit associated with identifying a safe and effective medical inhibition therapy for AAA disease.
Challenges Inherent in AAA Suppression Clinical Trials
Multiple logistical and scientific hurdles challenge the organisation and conduct of medical trials for AAA suppression. Some are common to clinical research in general, such as ensuring adequate recruitment, retention and patient adherence. Other challenges, unique to AAA trials, are outlined in Table 2.23 Notably, these include slow AAA growth, especially in the smaller, more common AAA that can obscure intervention efficacy, as well as loss to follow-up due to surgical repair (for larger aneurysms) and controversies regarding optimal endpoint assessment.
Perhaps the biggest challenge is uncertainty regarding the fundamental mechanisms of AAA disease initiation and progression. Most prior failed candidate mechanisms, including those outlined in Table 1, were imputed from the status of surgical specimens harvested at the time of operative repair: tissues typically atretic, relatively acellular, and of uncertain relevance to the initiating or sustaining conditions present earlier in the course of the disease, when drug therapy may be more effective. Additionally, problems with AAA experimental model systems limit their ability to provide independent, aetiological insight into the human condition. In humans, AAAs grow at a slow rate, approximately 2–3 mm per year, whereas induced model aneurysms dilate to rupture within days or weeks, implying that mechanisms of chronic aneurysm remodelling, for example, may not be well represented in experimental systems.36
Rationale for Trialling Metformin for AAA Disease Suppression
Existing Evidence: Bedside to Bench
Following decades of futility in translating aneurysm research into effective medical therapies, strategies are shifting to approaches identified through population science, rather than through animal modelling or pathological analysis of late-stage human tissue, to limit AAA disease progression. Unlike its influence on other peripheral CVD equivalents such as peripheral arterial or cerebrovascular disease, diabetes appears to reduce the burden of AAA disease, an observation that has intrigued investigators for more than 20 years.3 As first recognised in the US Department of Veterans Affairs Aneurysm Detection and Management (ADAM) trial, the concurrent diagnosis of diabetes not only reduces the risk of developing an AAA, but also reduces the rate of AAA enlargement and risk of AAA-related death when an aneurysm is present.37,38 Available evidence supports the conclusion that this benefit is not simply due to reduced life expectancy due to diabetes-related complications in affected patients.39,40
Mechanisms suggested to explain the protective effect of diabetes in AAA disease include reduced activation of pro-inflammatory macrophages in the extracellular matrix,41 modification of the balance of aortic mural pro- and anti-proteolytic enzymes,15 or advanced glycation end product-mediated limitation of mural proteolysis.42 Hyperglycaemia alone clearly limits AAA progression in experimental models.16 An alternative explanation, however, may lie in the medications used to manage hyperglycaemia in the older, insulin-resistant patient population also at risk for AAA disease.43
We examined the relationship of known aneurysm risk factors, comorbid conditions and diabetic and cardiovascular medications to the rate of aneurysm enlargement in AAA patients with diabetes identified from a clinical database of nearly 9 million patient visits to Stanford Health Care over a 10-year period.44 When entered into a logistic regression, after adjusting for known AAA risk factors, we found metformin therapy to be the variable most significantly associated with reduced aneurysm enlargement. In subsequent experimental modelling, we also found metformin to be effective in limiting AAA progression under normoglycaemic conditions in mice, underscoring the translational potential for metformin therapy even in the absence of diabetes. That study was the first to recognise and report the inhibitory potential of metformin on AAA progression in both diabetic (human) and non-diabetic (experimental modelling) conditions.44
In a subsequent report, Taiwanese investigators confirmed a negative association between metformin prescriptions for diabetes management and the diagnosis of AAA in their national health system. These investigators reported that the observed negative relationship was not a class effect: for example, present for metformin but not all hypoglycaemic drugs.45 Golledge et al. reported a negative association between prescription records for metformin and AAA disease progression in three separate small diabetic AAA cohorts in Australia, with adjusted ORs for a reduced likelihood of median or greater AAA growth of 0.59 (95% CI [0.39–0.87]), 0.38 (95% CI [0.18–0.80]), and 0.13 (95% CI [0.03–0.61]), respectively (all with p<0.02).46
Every retrospective study examining this question has reached the same conclusion: AAAs enlarge less rapidly in diabetic patients taking metformin versus those taking other hypoglycaemic agents (or those treated by dietary management alone), and diabetic patients taking metformin appear to be less likely to have concurrent AAA disease, accounting for all other relevant variables. Of the series reported to date, the negative association of metformin and AAA progression varies only in the size of the effect, ranging from 20 to 76% growth rate reduction compared with AAAs in diabetic patients not taking metformin, with the smallest effect size associated with the largest cohort (>10 000 patients).47 Importantly, this hypothesised inhibitory effect is significant even within a cohort of patients whose overall rate of AAA enlargement is significantly lower than that in patients without diabetes, with both clinical and experimental evidence suggesting that other features of diabetes, including hyperglycaemia,16 may also limit aneurysm enlargement.
For the first time in the history of aneurysm research, population science has identified a candidate therapeutic agent of great promise. This bedside to bench approach represents an innovative and promising new strategy for limiting AAA disease progression.
Novel Agent for Cardiovascular Disease Management
As noted above, comorbidities and concurrent medications present significant barriers to the conduct of meaningful AAA research. We recently participated in a multinational, multi-site trial of the angiotensin II receptor blocker (ARB), Study of the Effectiveness of Telmisartan in Slowing the Progression of Abdominal Aortic Aneurysms (TEDY; NCT01683084). The data supporting the use of telmisartan versus other ARBs in this application is compelling and previously summarised.20 The trial design itself was relatively straightforward, with well-defined endpoints and conservative sample size estimates.48 Yet TEDY struggled to reach sufficient power, largely because most eligible AAA patients were already on a regimen that included an ACEI or ARB, and to delete those medications from their overall regimen to facilitate trial enrolment would have been inappropriate and unethical. Similar problems have been encountered with alternative candidate agents such as statins and anti-platelet agents in prior AAA clinical trials.23 Essentially every drug used for CVD risk reduction is commonly prescribed in the setting of AAA disease, given that the latter is presumed to be a CVD equivalent, to reduce risk of all-cause mortality,42 making none of these drugs practical or realistic candidates for AAA suppression trials specifically.
There is intense interest in the ability of metformin to improve outcomes in cancer, cognitive disorders and cutaneous wound healing,as well as cardiovascular diseases.49–52 Indeed, people with type 2 diabetes (T2D) on metformin appear to have improved life expectancy compared with those managed with insulin or other insulin sensitiser agents.52
These data, as well as the large burden of obesity, metabolic syndrome, and cardiovascular disease in the US veteran population, recently led the Department of Veterans Affairs (VA) Cooperative Studies Program (CSP) to initiate a multicentre study to determine whether metformin therapy reduces the risk of major cardiovascular events (MACEs) in pre-diabetic patients (as determined by an HbA1c level from 5.7 to 6.5% in the absence of diabetes treatment) with established CVD (VA CSP 2002, NCT 02915198; Investigation of Metformin in Pre-Diabetes on Atherosclerotic Cardiovascular OuTcomes; VA-IMPACT). As of September 2019, this trial has enrolled more than 300 participants, with excellent adherence and drug tolerance reported to date.
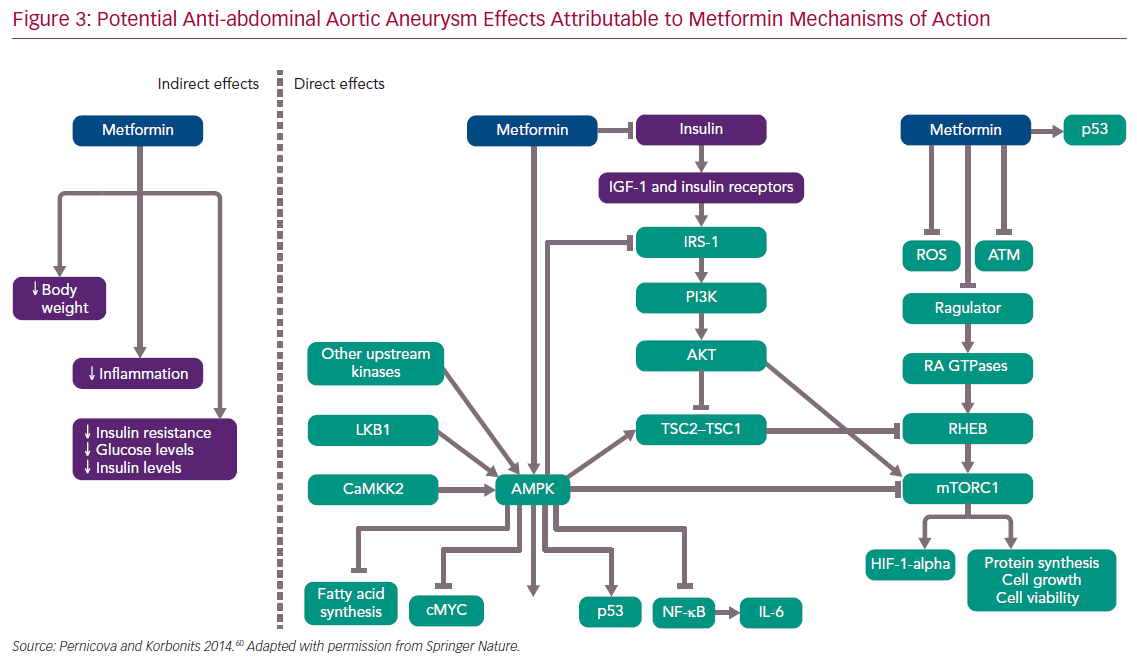
Despite widespread use, the precise mechanisms of action of metformin remain incompletely understood. In T2D, metformin, a weak inhibitor of the mitochondrial electron transport chain, increases intracellular concentrations of adenosine monophosphate, which in turn acts to lower blood glucose, enhance insulin sensitivity and favourably modify serum lipid profiles. In addition to indirect effects on vascular disease management (e.g. promoting weight loss and improved serum lipid profiles and endothelial function), metformin may limit the progression of AAA disease by inducing favourable effects on ROS production by infiltrative mural macrophages in atherosclerotic or aneurysmal vascular diseases, reduction of pro-inflammatory nuclear factor kappa activity, inhibition of the mammalian target of rapamycin pathway and autophagy, inhibition of mural angiogenesis (a key pathological feature of AAA disease), potential anti-inflammatory changes to the gastrointestinal microbiome, and upregulation of the silent information regulator 2 (SIRT) family of proteins or sirtulins, as partially demonstrated in Figure 3.19,22,53–60
Metformin therapy clearly reduces the burden of CVD, as measured by major adverse cardiovascular events (MACE) in people with diabetes.61 To date, however, no level I evidence has been generated regarding CVD endpoints, including AAAs, in non-diabetic patients. Given that nearly 50% of the American population over the age of 65 is in the pre-diabetes stage (and is also most at risk for sudden death due to AAA disease), the latter cohort remains of great interest in any proposed trial of metformin in AAA disease suppression. Although MACE as an endpoint captures potential death from AAA rupture, VA CSP 2002 does not assess for the presence or progression of AAA disease in trial participants, and will not lend insight into the potential influence of metformin on aneurysm pathobiology or progression of early disease (small aneurysm enlargement).
The influence of metformin on cancer is somewhat more controversial, with meta-analyses citing both salutary and indeterminate effects, leading to the initiation of multiple clinical trials of metformin in anti-oncologic applications, in patients with and without diabetes.62,63 Ample experience in cancer-related applications provides assurance that metformin therapy is well-tolerated and does not promote hypoglycaemia in non-diabetic or pre-diabetic patients except under unusual and well-recognised circumstances.64,65
In summary, given that no non-diabetic AAA patient is currently prescribed metformin outside the auspices of a clinical trial, this proposed trial design offers a highly innovative solution to enrolment hurdles that have hindered previous trials of broad classes of CVD risk reduction medications for AAA disease suppression.
Proposed Trial Design Considerations
Distinguishing Impact of Metformin Versus Diabetes Alone in AAA Suppression
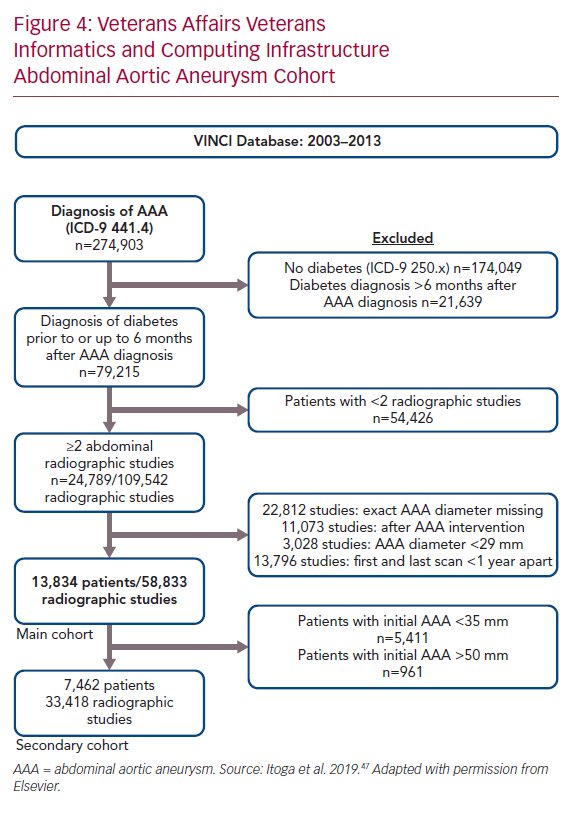
The original metformin AAA inhibition hypothesis was leveraged on retrospective case–control studies encompassing a few hundred patients, as described above. To gain more substantial perspective, we queried the Department of Veterans Affairs VA Informatics and Computing Infrastructure to identify all diabetic veterans with AAA disease treated nationwide between 2003 and 2013. Records were included in the study cohort if the diagnosis of diabetes was made prior to or within 6 months after the diagnosis of AAA disease; and patients had received at least two abdominal imaging procedures documenting infrarenal aortic diameter (ultrasound, CT, and/or MRI) in a ≥1-year interval between the first and last imaging procedure. Patients were censored from further analysis after undergoing surgical AAA repair. Aortic diameter measurements were obtained from radiographic reports.
The prescribed outpatient medical regimen at the time of AAA diagnosis (± 6 months) was obtained from pharmacy records. Patient comorbidities, smoking status, and other medication records were included from other VA online resources. Mixed effect modelling was used to fit the aneurysm growth rate (mm/year) to account for the inconsistent interval of radiographic dates and number of scans between individual patients.
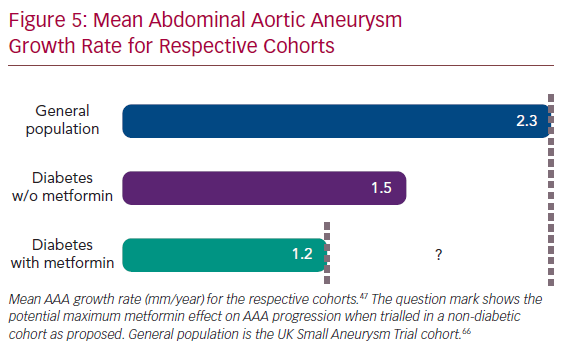
Using these methods, 13,834 diabetic AAA patients with 58,833 radiographic records were identified, with mean radiology imaging follow-up of 4.2 ± 2.6 years (Figure 4). The average patient age at diagnosis was 70 ± 8 years. Forty per cent had metformin prescriptions at or around the time of AAA diagnosis. In the study cohort overall, the average annual AAA rate of enlargement was 1.3 ± 1.6 mm/year, which was approximately 50% of the annual growth rate for AAAs identified on population screening in the UK Small Aneurysm Trial (UK SAT) cohort, of whom <5% had diabetes.66 The unadjusted mean rate of AAA growth was 1.2 ± 1.9 mm/year for patients with a metformin prescription compared with 1.5 ± 2.2 mm/year for the remainder (p<0.001); prescription for metformin was associated with a 20% decrease in yearly growth rate. When adjusted for comorbidities, this effect remained significant: a 0.20 mm/year reduction with metformin (95% CI [0.26–0.14], p<0.001; Figure 5). A secondary analysis of 7,462 patients with initial AAA size 35–49 mm (the size range appropriate for a clinical trial testing medical therapy) showed a similar decrease in AAA growth from 1.7 ± 2.2 to 1.4 ± 2.0 mm/year.47
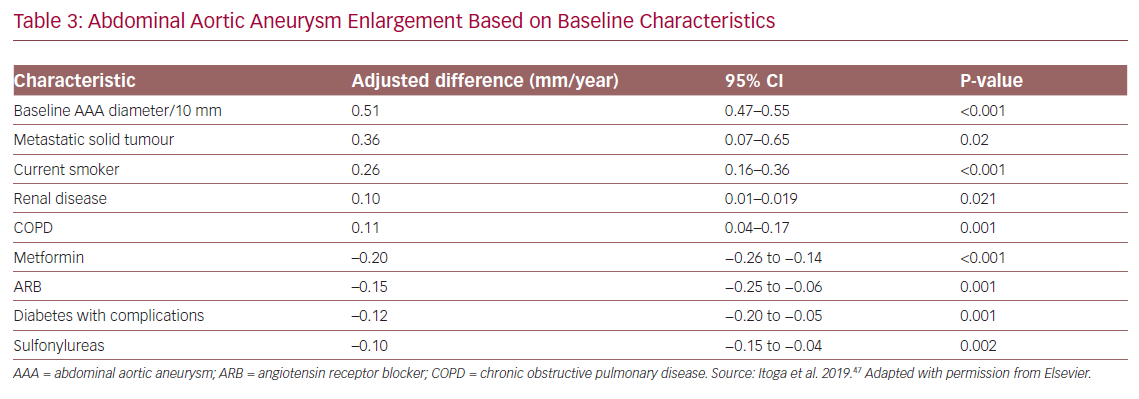
Factors associated with an increased AAA growth rate were baseline AAA size, metastatic solid tumours, current smoking, chronic obstructive pulmonary disease, and renal disease. Factors associated with decreased growth rates included prescription for ARBs or sulfonylureas and the presence of diabetes-related complications (Table 3).
These findings validate and extend those previously published by us as well as by others, all using different methods to address essentially the same question.
The association between diabetic complications and reduced AAA progression further supports the related hypothesis that increased chronic hyperglycaemia (reflected by diabetic complications) independently inhibits AAA progression, regardless of medical treatment provided, while refuting an alternative metformin explanation, for example that the association between metformin prescription and aneurysm suppression simply reflects the influence of more advanced diabetes. In practice, progressive insulin resistance and increased end-organ complications in T2D are managed with supplemental insulin rather than metformin in most cases, and no study to date has associated exogenous insulin therapy with AAA suppression. Recent observations also suggest that some AAA clinical benefits attributed to diabetes, metformin or both, such as reduced risk for surgical repair and rupture-related mortality, may be primarily attributable to metformin itself rather than to the underlying diabetic condition.67
Existing and Proposed Metformin Trials for AAA Suppression
In 2018, a pilot prospective, randomised, double-blind clinical trial testing the safety and efficacy of metformin to suppress early AAA disease in non-diabetic patients was initiated in Austria (Metformin Therapy in Non-diabetic AAA Patients [MetAAA], NCT03507413). Participants in this trial receive 12 months of drug therapy, with CT aortography (CTA)-determined rate of AAA diameter change between participants taking metformin and placebo as the primary study endpoint. Participants are prescribed metformin XR in 500 mg increments, up to 2000 mg/day, in a dose-escalating scheme to maximise tolerance and retention.
The MetAAA trial started in September 2018, and the estimated primary completion date is January 2022. In addition to clinical endpoints, MetAAA is comparing inflammatory cytokine profiles and markers of neutrophil activation in plasma between participants prescribed metformin and placebo. The proposed sample size is 170 participants (85 in each group) to achieve a power of 0.85. Early results suggest that metformin appears to be well-tolerated in this small group of older, non-diabetic AAA patients at short-term follow-up (verbal report from the principal investigator).
These investigators hope to use encouraging preliminary data from this pilot study, if and when available, to justify a much larger, pan-European consortium trial to more rigorously test the metformin hypotheses. As of September 2019, one additional metformin trial for AAA suppression has initiated enrolment in Europe (Metformin for Abdominal Aortic Aneurysm Growth Inhibition [MAAAGI], NCT04224051), along with an international trial being organised from Australia (verbal reports from the respective trialists). It remains to be seen whether any of these trials, proposed or running, will be able to effectively test the metformin hypothesis in an ethnically and racially diverse population burdened with high levels of obesity and sedentary lifestyles, characteristic of the middle-aged American population today.
Despite the somewhat cynical assessment that interest in metformin across such a wide spectrum of applications equates it to the “aspirin of the 21st century”, there is a clear and compelling opportunity now to validate metformin as the first pharmaceutical agent to effectively suppress progression of aortic aneurysm disease by proceeding with a well-designed clinical trial in the US.68,69 In preliminary modelling, we estimate that a 30% reduction in mean rate of enlargement for small AAAs (≥3.5 cm) with relatively inexpensive pharmacotherapy would reduce surgical utilisation and surgical costs by >US$23,000 per AAA patient over a 5-year period, as well as reduce AAA-related deaths in all patients by 40/1,000, or 4%. Based on the accumulated clinical evidence outlined above, clinical trials are justified at this time to confirm or refute the ability of metformin to prevent disease progression in non-diabetic AAA patients.
Experimental Approach
Two essential questions need to be answered to evaluate metformin’s suitability for this clinical application: is metformin therapy safe for, and well tolerated by, non-diabetic patients with AAA disease; and does metformin therapy suppress progression of small to intermediate-size AAAs in non-diabetic patients? The following proposed specific aims address these questions.
Specific Aim 1: Assess the Safety and Tolerance of Daily Metformin XR Therapy in Non-diabetic AAA Patients
Although metformin is currently being trialled extensively for anti-oncologic applications in non-diabetic patients, limited level I safety and tolerance data have been generated for non-diabetic patients with cardiovascular disease generally, let alone those with AAA (see the Existing and Proposed Metformin Trials section above). The most commonly reported side-effects in non-diabetic patients taking metformin are gastrointestinal in nature, infrequent and self-limited.
Hypoglycaemia is rarely experienced by healthy non-diabetic individuals taking up to 2,000 mg/day metformin, and occurs most commonly in debilitated and malnourished individuals, especially in the elderly, or in those with adrenal, pituitary or hepatic insufficiency. Metabolic acidosis is the most serious adverse effect associated with metformin use, and although infrequent, occurs mostly in patients with chronic renal insufficiency. Thus, patients with an estimated glomerular filtration rate (eGFR) <45 ml/min/1.73 m2 should be excluded from trial participation at the outset, and individuals whose eGFR falls below 30 ml/min/1.73 m2 should cease study drug ingestion. A comprehensive review of known health risks related to chronic metformin ingestion, important to any such trial design, is beyond the scope of this article.
The proposed trial (LIMIting AAA Progression with MeTformin; LIMIT) will recruit 480 participants, randomised 1:1 to metformin XR or placebo. For participants randomly assigned to metformin XR, daily dosage will begin at 500 mg/day and be titrated up in 500 mg increments/week to 2,000 mg/day over the first 4 weeks of the study (take one pill daily the first week, two daily the second week, etc.). Metformin tolerance will be assessed via quality of life surveys, laboratory monitoring, and analysis of participant adherence and retention metrics.
Specific Aim 2: Test the Ability of Metformin XR to Reduce the Rate of Enlargement of Existing Small to Intermediate AAAs by ≥30%, Compared with Placebo
This will be a prospective, randomised, placebo-controlled, double-blinded, stratified, Phase II superiority trial testing the ability of metformin to suppress progression of early AAA disease. In total, 480 non-diabetic participants with AAAs between 35 and 49 mm in diameter will be randomised 1:1 to metformin or placebo. A ≥30% reduction in the rate of annual AAA enlargement was chosen as a clinically significant translational target based on the review by Wang et al.70
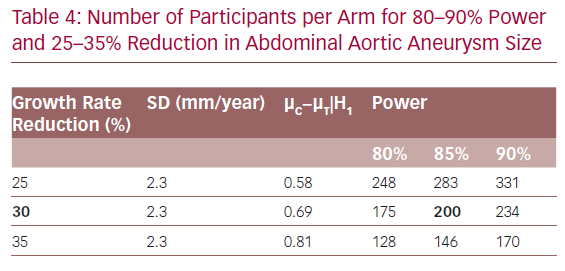
Sample size calculations are conservatively based on a two-sample t-test at a two-sided 5% significance level. Two hundred evaluable participants in each arm are required in order to have an 85% power to reject the null hypothesis of no difference when there is a 0.69 mean difference between two arms. Table 4 demonstrates sample sizes for a range of reductions and power levels.
To allow for a 20% dropout from the trial, we anticipate recruiting 240 participants per arm for a total of 480 participants enrolled. The projected dropout rate is conservative and based partly on the results of the Non-invasive Treatment of Abdominal Aortic Aneurysm Clinical Trial (N-TA3CT; NCT01756833), which required eight study visits over 24 months of study participation (versus five proposed for this trial), to determine if doxycycline will inhibit the increase of AAAs over a 24-month observation period.71 Dropout in the placebo arm of N-TA3CT was 19.5% (personal communication, Michael Terrin, N-TA3CT Co-PI, 27 February 2019). Notably, N-TA3CT was a multicentre trial, incorporating additional challenges in participant, staff, and centre retention and protocol adherence. At 480 participants, the current LIMIT trial design constitutes one of the most ambitious and comprehensive AAA growth inhibition trial proposed or conducted to date.
Potential participants will be recruited from Stanford Health Care (SHC), the Veterans Affairs Palo Alto Health Care System (VAPAHCS), and the Kaiser Permanente Northern California Health Care System (KP) in the greater San Francisco Bay Area of northern California. From 2006 to 2012, our research group conducted a single site trial of supervised exercise training for AAA patients at Stanford (Abdominal Aortic Aneurysm – Simple Treatment or Prevention; AAA-STOP).72 In AAA-STOP, more than 1,000 eligible patients with AAAs similar to those required for this trial were identified and screened from existing patient lists from the same health systems (SHC, VAPAHCS and KP).73 Given the substantially reduced requirements for participation in the proposed trial compared with three times per week supervised exercise training at a single location in the San Francisco Bay Area, as was required in AAA-STOP, we conservatively estimate that we can recruit 480 participants from the thousands of eligible patients in these combined registries.
Identifying eligible trial candidates from registries of existing patients is by far the most efficient method of recruiting potential participants for AAA-related clinical trials. In our prior experience, given the dearth of available treatment options for patients with early AAA disease, affected and eligible individuals are particularly eager to help identify effective alternatives to eventual surgical repair. In the unlikely event that registry-based recruitment does not suffice to meet trial enrolment goals, region-wide institutional review board-approved advertising will also be instituted to attract trial participants, trial participation will be promoted at local medical meetings, public interest events and health fairs, and commercial organisations such as LifeLine Screening will be contacted to identify additional candidates within the region.
The sex distribution of AAA disease is approximately 4:1 M:F. Enrolling subjects from all three systems will maximise our likelihood of obtaining a representative sample of sex distribution in our cohort (given that the VAPAHCS is predominately male). Given that the risk for AAA disease is age-related, and younger patients are likely to have syndromic aortic conditions that represent exclusions for study participation, eligibility is limited to individuals 55–90 years old. The upper age limit reflects the fact that participants need 2 years of follow-up to complete the trial.
Given that AAA disease affects mature individuals of all races and ethnicities, we will intentionally recruit a broadly representative trial cohort to maximise translational value of the derived results. Fortunately, the San Francisco Bay Area, home to more than 9 million individuals, is one of the most ethnically and racially diverse regions of the continental US. One-third of adults living in California were born outside the US. Again, our experience with AAA-STOP demonstrates that due to the substantial connection between cigarette smoking and AAA disease (much stronger association than AAA disease and ethnic/racial identity), we will be able to recruit from a broad and representative patient population, less skewed toward specific ethnic/racial identities than other areas of the US.74 According to ClinicalTrials.gov, no competing trials are enrolling patients with AAA of similar size in northern California at this time.
The primary study endpoint will be the relative rate of increase in maximum orthogonal AAA diameter through 24 months, as determined on CTA, in treatment versus control participants. All comparisons will be performed against placebo treatment, with stratification by sex, baseline diameter, smoking status (active or not), and HbA1c status (<5.7% versus ≥5.7% to 6.5%). ANOVA will be used to test the treatment effect controlling for stratification variables. Rate of AAA enlargement will be evaluated on CT angiographic measurement of maximum aortic diameter at baseline and at 24 months, and reported as a per-year rate (mm/year). Ultrasound measurement at 6-month intervals may be added as a secondary endpoint, both to add fidelity to the rate of growth and to capture some data from those who do not complete the 24-month scan. All measurements will be obtained by a core lab at Stanford with specific expertise in aortic aneurysm endpoint determination. Both manual and automatic measurement methods will be used, with examiners blinded to study group assignment.
Treatment consists of the highest tolerated dose of metformin XR (500–2,000 mg/day) for 24 months. Metformin will be paid for from the study budget. This trial is designed and powered to confirm the suppressive efficacy of metformin in a non-diabetic AAA patient cohort, if indeed it exists, as a Phase II trial. Broader generalisability of the derived results, if proven effective, should be provided by a subsequent Phase III trial adequately powered to assess optimal dosing regimens.
Maximum transverse aortic diameter (orthogonal when obtained from transverse abdominal CT) is the primary clinical standard to measure AAA disease progression, determine need for intervention, and correlate with clinical outcomes. Using this as the primary endpoint will insure the maximum translational relevance of the outcome. Reliance on outcome determination via serial CTA will also enable inclusion of exploratory endpoints including AAA volume, which although of uncertain clinical relevance in determining need for surgical intervention, may provide increased sensitivity in identifying an effect on aneurysm progression as noted in the Existing and Proposed Metformin Trials for AAA Suppression section. Also, high-resolution cross-sectional CTA will allow for quantification of peri-aortic adipose tissue volume present in the retroperitoneum at baseline and following 24 months on study drug between active treatment and control participants.
Recent observations suggest that increased circumferential peri-aortic adipose volume, as determined on CT, distinguishes AAA patients from those with aorto-occlusive disease or normal age-matched control aortic diameters,75 and genome-wide expression profiling of this adipose tissue, when harvested at surgery and compared between areas of maximal aortic enlargement and uninvolved proximal aorta, identifies an immunological signal consistent with underlying autoimmunity as a prominent influence in AAA pathogenesis.76 Given the known influence of metformin on weight loss and metabolic balance, an additional exploratory endpoint will include differential volume of peri-aortic adipose tissue in treatment versus control patients via CTA over the course of 24 months of study participation.
Rigor and Reproducibility
As discussed above and in Tables 1 and 2, best-practice solutions to the methodological challenges inherent in AAA suppression trials informed the design, methods and analysis plan of this proposed clinical trial. Probably the most significant reproducibility consideration for this trial is its single-centre design, intentionally adopted to minimise the variability in eligibility assessment, data acquisition, participant follow-up, and study adherence inherent in multicentre constructs. Although recruiting from phenotypically well-characterised AAA cohorts compiled from three distinct regional healthcare systems to maximise enrolment, all trial activities take place at the Stanford Clinical and Translational Research Unit (CTRU) to ensure uniformity of data acquisition and participant engagement.
Study eligibility is based on the confirmed presence of an AAA in the diameter range deemed appropriate for trial participation, excluding syndromic or traumatic aneurysms. To maximise the likelihood of progressive AAA enlargement in all participants, only patients with larger AAA (those between 3.5 and 4.9 cm in diameter) will be included. The selection of CTA-determined aortic diameter rate of change as the primary clinical endpoint will minimise imprecision and interpretation error. Comprehensive accounting of, and outcome stratification based on known confounding conditions and comorbidities in AAA disease will maximise accuracy and reproducibility of the determination of a metformin effect.
Conservative assumptions and definitions have been applied to the target effect size and determination of significance to maximise rigor and reproducibility. Working within the auspices of the CTRU ensures rigid adherence to protocol and timelines. Real-world and disease-specific assessment modalities, such as CTA and AAA-specific quality of life surveys, were incorporated wherever possible to maximise translational impact of the acquired results. Even the selection of metformin as the candidate inhibitor was made, in addition to potential efficacy considerations, with reproducibility considerations in mind (see the Existing Evidence section above). Also, the broad outline of our study protocol has been shared with collaborative groups worldwide in an effort to allow data aggregation for maximum sample size and power should all currently proposed trials be completed (see the Existing and Proposed Metformin Trials section).
In short, all aspects of trial design, methods, data analysis, and results reporting (including adaptation of rate of orthogonal AAA diameter enlargement, the primary determinant for risk of AAA rupture, as the primary study endpoint) were adopted with the goal of maximising the rigor and reproducibility of this proposed trial.
Conclusion
After decades of research and hundreds of thousands of premature deaths from untreated or undertreated AAA disease or complications from surgical repair, this proposed trial provides the opportunity to validate metformin as the first inexpensive, non-toxic, and effective pharmacological agent to reduce the burden of AAA disease worldwide.