Materials and Methods
From October 2001 to April 2003, all patients with AAAs > 5cm, with or without concomitant iliac artery aneurysms, underwent thin-cut contrast-enhanced computed tomography scans in evaluation for open surgical repair or EVAR. Only patients with infrarenal AAAs were included in this analysis. All pararenal or juxtarenal aneurysms having open surgical repair were excluded from this study. EVAR was performed with a variety of stent grafts (AneuRx®, Ancure®, Excluder® and Lifepath®), and open surgical repair was performed via a retroperitoneal approach. 6 Patients were divided into two groups based on the type of repair: endovascular (Group I) or open surgical (Group II). Data was collected in the vascular registry and analysed for demographics, perioperative morbidity and mortality and hospital length of stay. Data comparison between the two groups was achieved by using chi-square analysis and two-tailed student’s t-test. Statistical significance was identified at P<0.05.
Results
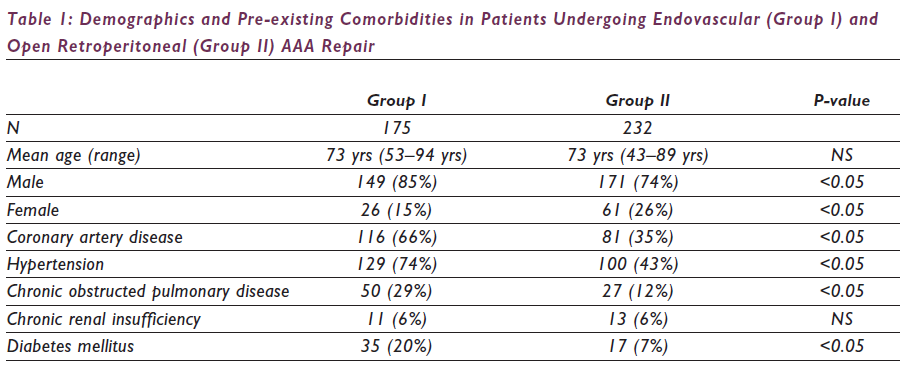
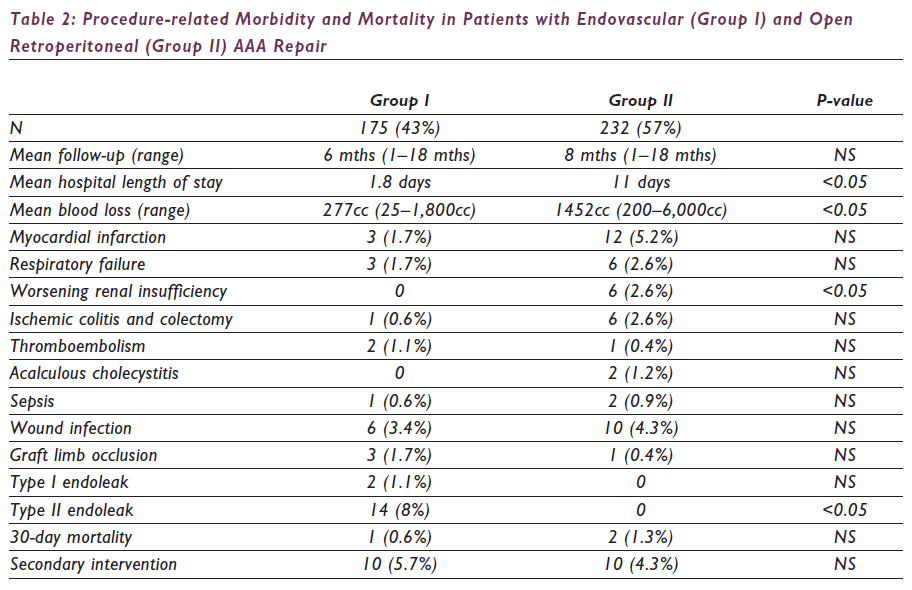
Over the 18-month study period, 446 patients presented with asymptomatic (n=372), symptomatic (n=36) and ruptured (n=39) infrarenal AAAs and underwent repair via an endovascular (n=186, 42%) or open retroperitoneal (n=260, 58%) approach. Patients presenting with ruptured AAAs were excluded from the study, and the remainder were divided into two groups based on the type of repair: endovascular (Group I: n=175, 43%) or open retroperitoneal (Group II: n=232, 57%) approach. The demographics and risk factors are listed in Table 1. Post-operative morbidity and mortality are listed in Table 2.
All patients with graft limb occlusions underwent successful thromboembolectomy. Group I patients required additional iliac stent graft extensions. One Group I patient (0.6%) and three Group II patients (1.3%) died of myocardial infarction and multisystem organ dysfunction, respectively. The mean follow-up for Groups I and II is six months (range: 1–18 months) and eight months (range: 1–18 months), respectively. Sixteen (9%) Group I patients developed endoleaks from proximal fixation sites after stent graft migration (Type I: 2, 1%) or from retrograde flow through the lumbar and inferior mesenteric arteries (Type II: 14, 8%). Both patients with Type I endoleaks underwent successful proximal aortic stent graft extensions without any evidence of persistent Type I endoleak. Two patients with Type II endoleaks and an increase in aneurysm sac diameter underwent translumbar coil embolisation of the endoleak channels and aneurysm sac with a resultant decrease in mean sac pressure. The remaining 14 patients with Type II endoleaks have been treated expectantly.
Discussion
The objective of AAA repair is to prevent rupture with a limited morbidity and mortality. EVAR offers a less invasive means of treating AAAs, and several reports have suggested a decreased post- operative stress response, morbidity and hospital length of stay when compared with open surgical repair through a transperitoneal approach.10–12 In our experience, the retroperitoneal approach offers advantages over the transperitoneal approach in open aneurysmorrhaphy. Therefore, EVAR was compared with a standard retroperitoneal repair of an infrarenal AAA. Our data suggests that, regardless of any increased pre-existing comorbidities, patients undergoing EVAR have significantly less blood loss, less worsening of renal function and a shortened hospital length of stay when compared with patients undergoing conventional open repair with the retroperitoneal technique. However, there are no significant differences between the two groups with regards to respiratory failure, wound infections, graft limb occlusion, myocardial infarction, ischaemic colitis, thromboembolism or death.
In this retrospective analysis, there were several differences between the two groups, some of which could be attributed to our selection biases. Patients undergoing EVAR had a significantly higher incidence of pre-existing comorbidities of coronary artery disease, hypertension and chronic obstructed pulmonary disease. The incidence of pre-existing renal insufficiency was similar in both groups without significant worsening of renal function in the endovascular group. To minimise the adverse effects of chronic renal insufficiency in our patients, we routinely discontinued all possible nephrotoxic drugs, hydrated pre-operatively with normal saline, infused intravenous mannitol (0.5gm/kg) intraoperatively, preferentially used non-nephrotoxic contrast agents such as gadolinium and carbon dioxide and limited the volumes of any low-osmolar contrast agent.13 The incidence of women undergoing AAA repair was lower in the endovascular group (15% vs. 26%). This was probably a result of morphological gender differences and the inability of currently available stent grafts to accommodate aortoiliac aneurysms in women.14
Technical success in Group I was obtained in 99.4% (175/176) of the patients. In one patient, we were unable to obtain access through calcified, stenotic and tortuous iliac arteries, resulting in our inability to perform EVAR. Nine (5%) high-risk patients with complex aortoiliac aneurysms had an aorto-uni-iliac stent graft and a fem-femoral bypass with contralateral hypogastric artery interruption (HAI), and seven (4%) patients had bilateral HAI and stent graft extension onto the external iliac artery. Although historical data on bilateral HAI during open surgical repair suggested a high incidence of complications from pelvic ischaemia, our experience suggests that bilateral HAI during EVAR is associated with a limited morbidity. With bilateral HAI, buttock claudication can develop in up to 40% of patients. However, in our experience to date of evaluation of 46 patients with bilateral HAI, the incidence of impotence (12%), ischaemic colitis (3%), buttock necrosis (0%), neurological deficits (0%) and death (0%) suggests this to be a relatively benign procedure as long as the collateral pelvic circulation is preserved and distal emboli avoided.15–16 In patients undergoing EVAR, the overall complication rate and severity were less when compared with the open retroperitoneal repair. Substantial differences were noted in the incidence of post-operative myocardial infarction, ischaemic colitis requiring colectomy and worsening renal insufficiency. Open retroperitoneal repair was associated with increased operative blood loss, haemodynamic instability and stress response, which can lead to the increased incidence of post- operative complications.
Over a six-month follow-up, two (1%) patients developed a Type I endoleak from the proximal fixation site, and 14 (8%) patients developed a Type II endoleak. Both Type I endoleaks resulted from stent graft migration and were treated with the addition of aortic cuffs. Two (14%) patients with Type II endoleak were noted to have an increase in the maximum aneurysm sac diameter and underwent translumbar sac puncture and successful coil embolisation of the lumbar arteries, resulting in a decreased aneurysm sac pressure. None of these patients had any complications with these procedures. There is no significant difference in the need for secondary interventions or 30-day mortality between the two groups.
In conclusion, despite increased pre-existing comorbidities, patients undergoing endovascular aneurysm repair have significantly less blood loss, hospital length of stay and renal insufficiency, even when compared with open retroperitoneal aneurysm repair. Although long-term data is lacking, the results of minimally invasive EVAR are promising in carefully selected patients with anatomic suitability. It is imperative that patients understand the palliative nature of this procedure and be willing to undergo vigilant routine lifetime follow-up.