Vascular closure devices (VCDs) were introduced into clinical practice in 1994. The purpose of these devices was to reduce vascular complications after percutaneous femoral access, provide rapid haemostasis and early ambulation and improve patient comfort. These devices are frequently part of clinical practice, with millions implanted annually. However, their success has been mixed. Worldwide, fewer than half of invasive procedures utilise VCD and there are strong advocates for alternative approaches (manual compression or radial artery access).1 Compare this situation with that of another technology introduced around the same time – coronary stents. Fifteen years after the introduction of this breakthrough technology, stents have nearly entirely replaced the previous approach (balloon angioplasty). While there are passionate debates about which stent to use, there are few clinical arenas in interventional cardiology where the superiority of some type of stent implantation is not accepted. Stents (especially drug-eluting stents) cost five to 10 times more than VCDs and have a more devastating adverse event risk (stent thrombosis), and neither device patently saves lives compared with older approaches. This rather divergent history of two interventional cardiology technologies over the past 15 years provides an opportunity to ask the hard question: why have VCDs not become the default approach for managing femoral access?
Vascular Access and Complication Rates
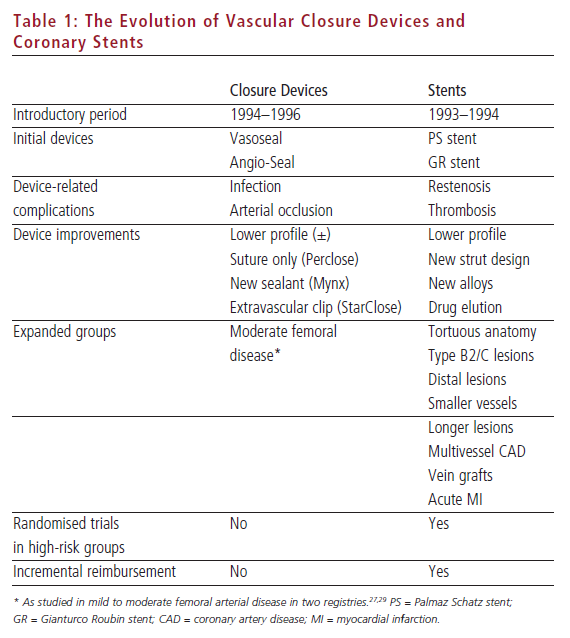
Stents were introduced with two landmark clinical trials showing clear superiority with respect to reduction in an important non-mortality end-point: angiographic and clinical restenosis (see Table 1).2 VCDs were also introduced showing a reduction in a non-mortality end-point: requirement for prolonged bed-rest.3 Later studies of VCDs also addressed secondary end-points of improved patient comfort and time to discharge.4 Both new technologies created a scenario for new and serious complications: stent thrombosis5 and acute femoral arterial compromise or infection.6–9
However, VCD adoption was far more sporadic than that of stents. Three reasons can be identified:
- While VCD cost is far less than that of stents, within a short time period stents are more financially viable. On the other hand, VCD implantation has not been reimbursed (in the setting of inpatient diagnosis-related group [DRG] systems) and cost-effectiveness depends on multiple factors, including plans for early discharge.1,10,11
- Clinicians recognised the importance of multiple secondary end-points but placed an appropriate emphasis on the end-point of vascular complications: VCDs were quickly associated in meta-analyses and case reports with increased risk of vascular complications,12 and this too prevented widespread adoption.
- The major drawback to stenting was the risk of early 30-day stent thrombosis; however, the interventional community quickly addressed and reduced this risk with the introduction of dual antiplatelet therapy and high-pressure stent deployment.13 On the other hand, subsequent VCD introduction in the late 1990s appeared to have a less dramatic impact on the risk of vascular complications.1,12,14 Thus, the interventional community was divided: some placed a high value on patient comfort and early ambulation, while others were cautious adopters of VCDs in the setting of unclear reduction in vascular complication rates.
This equipoise with respect to VCD utilisation has led to two themes over the past five years. First is an emphasis on an alternative approach in which femoral access obviated radial artery access. Similar to VCDs, this approach was introduced at the same time as coronary stents and consequently adoption has been even less than that of VCDs.15 While there is great regional variation in the utilisation of the radial artery approach worldwide, the overall utilisation in international clinical trials of radial access remains lower than 10% of all procedures.16 Given the potential superiority of the radial versus femoral approach with respect to vascular complications, the interventional community has once again spoken: new approach/device utilisation is sometimes dictated not by primary or secondary end-points; instead, the tertiary end-point is defined as ease of use and procedural time remains an important determinant of clinical adoption.
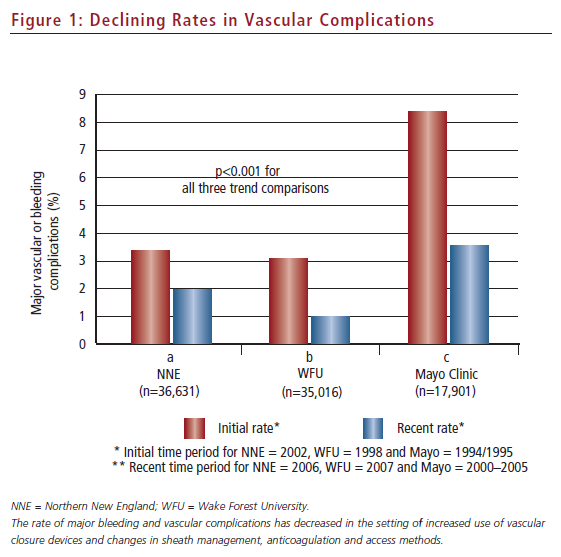
The second theme to emerge over the past few years has been a re-analysis of the vascular complication rate in the setting of VCD utilisation (see Figure 1). This has occurred in the context of improved femoral access techniques and attention to appropriate utilisation of VCDs based on routine femoral angiography performed prior to VCD implantation.17,18 Four groups have now examined recent trends in vascular/bleeding complication rates in the more recent era of increased VCD utilisation. First, an analysis by Tavris et al. of the National Cardiovascular Data Registry (NCDR) demonstrated no increased risk of vascular complications with VCDs and, in fact, suggested a reduction in complications in patients receiving VCDs after undergoing diagnostic catheterisation.6 Second, registry studies from the Northern New England Cardiovascular Study Group, the Mayo Clinic and Wake Forest University Medical Center have demonstrated that vascular complications are significantly lower now than they have been at any time in the history of interventional cardiology.1,19,20 Thus, an opportunity now presents itself for VCDs to assume a more stent-like history: by addressing the concerns of all secondary end-points (ease of use, procedural time, early ambulation, early discharge and an improving profile of vascular complications), there may be only two major barriers remaining to broader adoption, i.e. reimbursement and better technology.
Better Vascular Closure Device Technology
To return to the stent analogy, no one uses the original Palmaz Schatz or Giantorco Roubin stents anymore. In fact, these cumbersome (Palmaz Schatz) and inferior (Gianturco Roubin) stents were quickly replaced by more deliverable and effective platforms that allowed expansion of stent adoption to more difficult coronary anatomy and higher-risk patient subsets. Compare this with the history of VCD development and adoption: while the first VCD (Vasoseal) has been largely eliminated from practice due to consistent concerns about increased risk of complications, the second VCD (Angio-Seal) has undergone moderate modifications14 and remains the market leader in utilisation. While stent companies have utilised the ‘one new stent a year’ approach to market-driven improvement in deliverability and, more recently, restenosis rates, VCD development has been slower. Most importantly, VCD development has not clearly expanded the patient pool eligible for closure devices (see Table 1).
Thus, while broader adoption of stenting in difficult lesions was demonstrable in newer-generation stents, VCD adoption in higher-risk situations has focused on certain clinical and pharmacological situations,21,22 while other areas – i.e. peripheral vascular disease, non-femoral artery punctures, calcified vessels – have not been thoroughly investigated.
Better VCD technology and broader application to higher-risk groups may be a major driver to the potential stent-like adoption of new VCD technology. One theme that has recently emerged is re-visiting the initial concept of VCD embodied in the Vasoseal device: extravascular closure. Specifically, the ideal VCD would not have any intravascular components of polymer (Angio-Seal) or suture (Perclose) that may potentially lead to femoral artery compromise via mechanical effects or inflammatory reactions.23 Notably, the original extravascular closure device, Vasoseal, is a failure. It is the one device associated with a significantly increased risk of vascular complications in registry studies.6 Thus, the inherent superiority of an extravascular to an intravascular closure device cannot be assumed.
Nevertheless, the concept of extravascular closure with no remaining foreign body in the artery is attractive to the interventional community. This attractiveness is theoretical and analogous to the concept of biodegradable polymers as a potential solution for late stent thrombosis. This extravascular closure concept may be especially attractive in the setting of patients requiring access to diseased femoral arteries24,25 with potential for device dislodgement and arterial compromise.
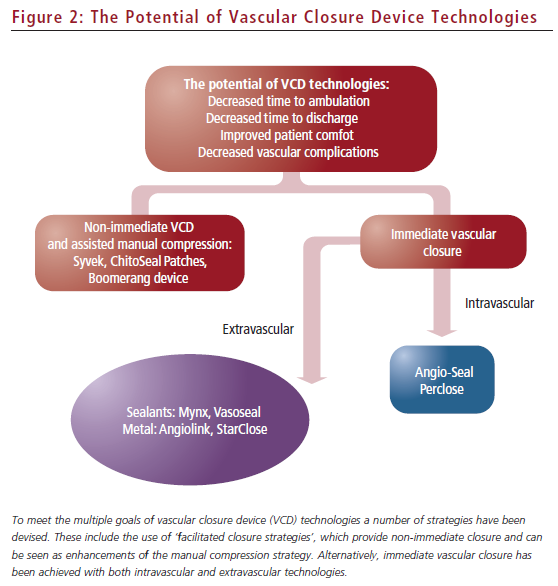
There are multiple approaches to achieving immediate haemostasis via extravascular closure (see Figure 2). For example, staple- or clipmediated closure, similar to that performed in closure of surgical incisions, is one attractive concept. The first metallic extravascular closure device studied, Angiolink, showed potential efficacy even among patients with higher-risk femoral anatomy.26,27 The second metallic extravascular closure device studied is the nitinol clip-based closure device, StarClose.28 This device has shown efficacy and met the earlier secondary end-point concerns (early ambulation, earlier discharge and no increase in vascular complications) of the interventional community in the initial trials compared with manual compression. StarClose has also been studied in a dedicated registry of higher-risk patients undergoing femoral access, with promising results.29 In this study of 98 patients with higher-risk (but not severe) femoral arterial disease, complication rates were low and time to haemostasis was less than one minute on average.
However, these extravascular metallic closure devices are not perfect. First, neither has been studied or should be currently used in patients with severe femoral artery disease due to concerns about stapling/clipping the anterior and posterior walls of the artery together (or dissection at the time of locating the arteriotomy in a small diffusely diseased vessel). Second, they do not always work: for example, in the Closure In Percutaneous Procedures (CLIP) trial, one can find approximately 10% of patients who require prolonged manual compression due to failure to obtain immediate haemostasis.28 While the learning curves for these newer devices may be less than for the intravascular-based Perclose device,24 some element of a learning curve can be assumed and it is possible that efficacy will improve over time. Third, no device has been studied in patients at risk of one of the most devastating vascular complications: retroperitoneal bleeding (which is associated with sheath insertion above the inferior epigastric artery).30
There are alternative closure strategies that have also been developed over the past decade (see Figure 2). Extravascular sealants (Mynx device) have re-emerged as an immediate closure option.25,31 This sealant is different from the one used in Vasoseal and thus may not encounter the same problem of enhanced bleeding complications observed with that device. However, broader study, especially in high-risk patients, has not been presented to date, so the potential for this device to increase the adoption of VCDs in higher-risk groups remains to be determined. Second, delayed-closure devices may have a role in providing haemostasis with early ambulation, although not with the immediacy of the Angio-Seal, Perclose or StarClose devices.32,33 Thus, these ‘facilitated closure’ devices may follow the pattern of the radial artery access adoption in which the tertiary concerns of ease and speed of use may limit their utilisation by many practitioners.
From Better Technologies to the Best Vascular Closure Devices
Comparisons of VCDs are limited by the low vascular complication rates inherent in initial randomised clinical trials of new devices. Demonstrating a 50% reduction in complication rates with one device compared with another could require enrolment of thousands of patients if the event rates are in the range of 1–2%. One option for future comparisons is to focus on high-risk patients (i.e. those with peripheral vascular disease), in whom the event rate would be expected to be at least 4–5%,34 and thus a lower enrolment number would be required. To date, such a study has not been carried out.
Can we define a single best technology among the current VCD products? Registry studies have compared Angio-Seal versus StarClose with inconclusive results. For example, in one non-randomised comparison of approximately 500 patients, major vascular complications were seen in 2.9% of Angio-Seal, 1.9% of StarClose and 3.7% of manual compression patients, with no significant difference demonstrated;35 previous meta-analyses have been inconclusive in comparing different devices with the exception of an increased risk with vasoseal.1 Two recent randomised trials comparing Angio-Seal and StarClose have also failed to identify a clear ‘winner’: in one study, StarClose was associated with more oozing, and in the other Angio- Seal was associated with more late bruising complications.36,37 Comparisons of local inflammatory responses in canine models have suggested an advantage of Perclose over Angio-Seal,23 but systemic differences in inflammatory response have not been seen.38 A porcine study comparing the arterial injury and inflammatory response to Angio-Seal versus StarClose has suggested an early detriment to Angio- Seal but equivalence at 60 days.39 Thus, we lack clarity regarding the clear superiority of a single VCD at this point.
Each device currently on the market has advantages, drawbacks and potential concerns. Progress has been made since 1994 with respect to VCDs, but the dramatic evolution of coronary stents has not been entirely seen in the VCD world. For example, imagine that you had a 1994-era coronary stent that failed to deliver in 10% of cases, caused life-threatening thrombosis in 3–5% of patients despite use of multiple anticoagulants, required use of a 7- or 8-French sheath, could not be delivered distally and was limited by a 20–30% restenosis rate – would you use it in nearly 100% of patients? The answer is no. The universal adoption of stenting has occurred in the setting of major changes: new flexible designs, lower profiles, cobalt–chromium deliverability, drug elution and dual antiplatelet therapy.
What would a similar change in VCD development look like? The best VCD would provide immediate closure in over 98% of patients, decrease vascular complication rates to a new low of 1% and apply to patients with difficult anatomy in which femoral artery compromise/ dissection are of concern. In addition, unlike the radial artery approach, the device would be easy to use, with a learning curve of fewer than 25 cases, and would be fast to deploy. To achieve these results and to achieve uniform adoption of VCDs in a fashion analogous to stenting, the VCD industry will need to look to new alloys/coatings and sealant methods, less lumen compromise, lower-profile technology, better haemostasis mechanisms and definitive expansion to higher-risk patients. Based on the improving technology and complication rates over the past decade of VCD development, we can expect to see an increased adoption of VCDs to the point now at which the majority of procedures may utilise VCDs. However, VCDs will not become a uniform standard of care until we truly define a ‘best technology’ by initiating a ‘stent-like’ development to move us well beyond the VCD platforms of the 1990s.