Wearable technology refers to the broad category of compact electronic devices that can be incorporated into clothing or accessories.1 These devices are used in the clinical and non-clinical environment and they offer multiple applications that help maintain physiological and psychological wellbeing.2 Wearable technologies not only facilitate self-tracking for the consumer but also allows for remote monitoring and analysis by a third party, such as a healthcare provider.
The Internet of Things is a network of real-world objects that have the ability to communicate data and sense the status of each object and the surrounding environment.3 Through the combined use of wearable devices and Internet of Things technology, patients and healthcare providers may be able to track, monitor and analyse various clinically relevant measurements. This could lead to real-time monitoring of disease progression as well as treatment adherence.
This concept feeds into the premise of telemonitoring, which is defined as the use of telecommunication devices to remotely monitor patients at a distance.4 Of note, telemonitoring is broadly synonymous with biotelemetry, which involves the transmission of health data from one location to another where the data can be interpreted and used to affect healthcare decision-making.4,5 Telemonitoring is useful in collecting biological, environmental or physiological data in a remote setting when direct observation is not possible.5 Common clinically pertinent measurements such as blood pressure (BP), blood glucose and lung function have been successfully tracked using telemonitoring technologies.6–10 The use of telemonitoring allows patients to be managed at home, thereby helping to overcome barriers concerning access to healthcare, such as travel, time and costs.
These technological advancements may prove useful in the management of cardiovascular disease (CVD). CVD is a major global health burden and accounts for a significant proportion of hospital admissions, as well as up to one-third of all deaths globally.11 However, given the importance of conservative management and lifestyle modifications, wearable technology may be able to disrupt the way in which medical therapy and lifestyle modification advice is delivered to this patient cohort. Therefore, in this review, we aim to summarise the current applications of wearable and telemonitoring technologies in facilitating the management of CVD outside the hospital setting.
Current Wearable Technologies
The broad definition of wearable technologies encompasses a variety of devices, such as head-mounted displays, clothing with smart technology, fitness trackers, smart watches and biosensors.
Fitness trackers (activity monitors) allow the long-term daily monitoring of physical activity in a real-world setting and usually take the form of wrist bands, ankle bracelets or clip-ons. Fitness trackers use sensors (pedometers or accelerometers) to detect movement and are therefore able to measure physical activity. Electronic displays on devices allow quick and easy viewing of exercise progress and goals. Many devices are able to transfer data to computer or smartphone apps, allowing remote access to data.
Luley et al. used AiperMotion 440 (Aipermon) fitness trackers as part of an intervention to increase physical activity in patients with metabolic syndrome and found significant improvements in weight loss and markers of the metabolic syndrome.12 In a randomised controlled trial, Frederix et al. used triaxial accelerometers (Yorbody) to monitor physical activity and set daily step-count prescriptions for patients with coronary artery disease enrolled in hospital-based cardiac rehabilitation.13 This study found a significant improvement in lung function assessments and a trend towards fewer rehospitalisations in the intervention group. Kirk et al. conducted a meta-analysis examining the use of wearable devices to alter physical activity behaviour in adults with chronic cardiometabolic disease and found a positive impact on physical health.14
Smart watches have many of the same functions as smartphones, such as mobile applications, internet connectivity and GPS.15 Similar to fitness trackers, smart watches can also incorporate movement and exercise sensing functions, but their functionality is expanding. For example the Apple Watch (Apple) is currently being validated by clinical trials for detection of AF and other abnormal heart rhythms.16
Wearable biosensors are attached to the body for long continuous periods of time and have diagnostic and monitoring applications. The Wearable Biosensor (Philips Medical Systems) is a lightweight, wireless self-adhesive biosensor that can detect vital signs – ECG, respiratory rate, skin temperature – and movement data – posture, fall detection, step count. A study conducted by Braem et al. found that the use of this wearable device as a tool for screening activity levels in patients undergoing transcatheter aortic valve implantation was feasible, but noted some concerns regarding the reliability of the data collection.17 Continuous glucose monitoring can provide real-time blood glucose data otherwise unobtainable by conventional intermittent sampling. GlucoWatch (Cygnus) is a non-invasive wearable continuous glucose monitor that extracts and samples glucose through intact skin via reverse iontophoresis.18 A validation study by Tierney et al. found blood glucose readings from the device were clinically acceptable. However, a randomised trial by Chase et al. found no improvement in glycaemic control with the GlucoWatch, which was, in part, attributed to poor device adherence due to skin irritation.18,19
Peripheral Artery Disease Management
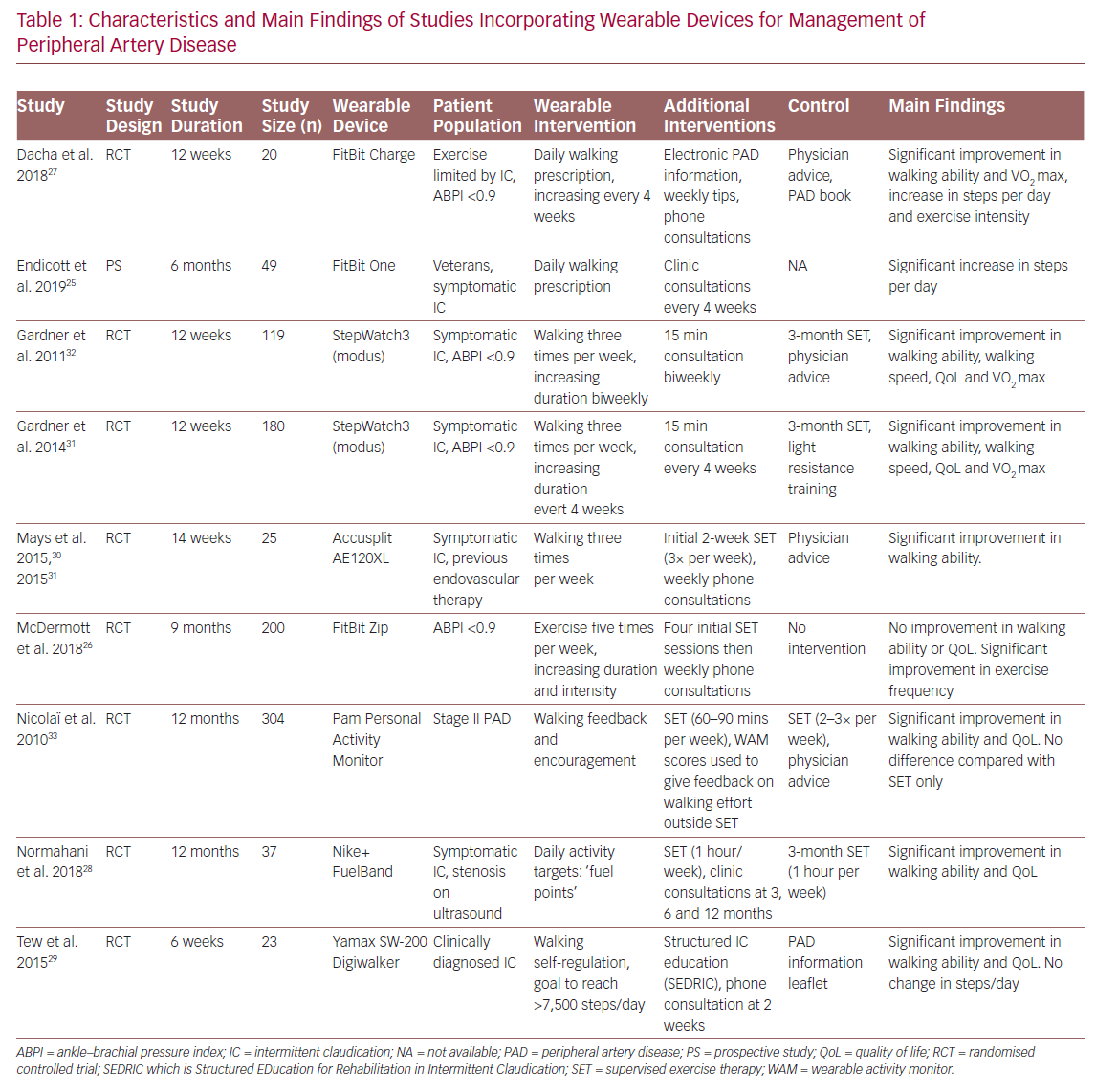
Wearable devices with the ability to monitor movement may be particularly useful in the management of patients with peripheral artery disease (PAD). PAD is a debilitating condition that results in significant walking impairment and poor quality of life.20 Current recommended first-line management for PAD is supervised exercise therapy, with patient mobilisation crucial to prevent disease progression and reduce hospital admissions.21,22 Despite this, patients often face challenges in accessing therapy due to cost, travel and time constraints. This contributes to the low uptake and adherence with such programmes.23,24
Several studies have investigated the efficacy of home-based exercise programmes incorporating fitness trackers and telemonitoring as an alternative to supervised exercise (Table 1).25–33 Duscha et al. used wrist-worn commercially available fitness trackers (FitBit Charge, FitBit) to monitor daily physical activity and set exercise prescriptions based increasing patients’ step count each day.27 Patients wore fitness trackers continuously during waking hours and daily step count was synchronised with smartphones where it could be remotely viewed. This allowed investigators to not only monitor physical activity, but also offer personalised feedback via phone calls. Patients using the fitness tracker intervention were found to have significant improvement in claudication distance, maximum walking distance and steps walked per day, compared with baseline.
Similarly, Normahani et al. incorporated the Nike+ FuelBand (Nike) fitness tracker as part of a home-based exercise intervention for patients with PAD.28 Instead of setting exercise prescriptions using step count, ‘fuel points’ were used, which measure overall activity and movement. Online accounts on the Nike+ website allowed activity data to be reviewed at follow-up visits and exercise prescriptions could be programmed into patient devices. The study found significant increases in walking ability and quality of life in patients using the device, compared with those attending a weekly 1-hour supervised exercise session over a 12-month period.
In addition to using the AE120XL pedometer (Accusplit) to track daily physical activity and weekly phone consultations, Mays et al. incorporated an element of coaching into their study.30 This allowed them to identify local barriers and encourage exercise adherence. This involved an assessment of a patient’s local area using Google Street View to identify walking routes and community resources, such as benches on which to rest. Moreover, they identified potential barriers to walking, such as discontinuous pavements.30,34 This resulted in significant improvements in walking ability for the intervention group compared with baseline and usual care.
The use of smartphone applications may also be beneficial for the remote monitoring and management of PAD. Ata et al. developed VascTrac, an Apple smartphone app that enabled remote collection of medical and physical activity data.35 VascTrac is able to record measures of daily physical activity (steps walked, distance walked, flights of stairs climbed and maximum continuous number of steps) using the built-in accelerometer in Apple smartphones. Patients were sent notifications through the app to perform twice-weekly 6-minute walk tests which allows tracking of changes in walking ability. Study results are yet to be published.
Blood Pressure Monitoring
Hypertension is a well-known risk factor for CVD.21 Environmental factors can often limit the usefulness of conventional BP measurements because of phenomena such as white-coat, masked and nocturnal hypertension.36 Home measurement is a superior method of determining BP and wearable devices are well established for this purpose.37 Apart from traditional oscillometry-based BP measurement (involving an inflatable cuff), wearable devices also use other non-invasive methods of eliciting measures of BP such as tonometry, volume clamp, pulse wave velocity and pulse transit time (Table 2).38
The BPro (Healthstats) is a cuffless, wrist-bound BP measurement system that works via arterial applanation tonometry. The wrist monitor incorporates a small protruding force sensor that rests over the radial artery and captures the arterial pulse waveform.39 The device requires initial calibration to bronchial BP using a standard oscillometry-based BP monitor. In a validation study, the device was found to meet the accuracy criteria for systolic and diastolic BP in both European Society of Hypertension protocol and Association for the Advancement of Medical Instrumentation (AAMI) standards under stationary conditions.40 However, in a study comparing accuracy of the BPro against intra-arterial BP measurement in post-operative patients, there were significant inaccuracies according to AAMI standards in systolic and mean BP between the methods, which was attributed to patient movement.41 A 24-hour study comparing the BPro to conventional arm-bound oscillometry-based BP monitoring in 50 normotensive and prehypertensive volunteers found fair agreement between the two devices.39 However, during BP measurements the volunteers were asked to keep still and the BPro was unable to obtain almost 50% of the scheduled measurements.39
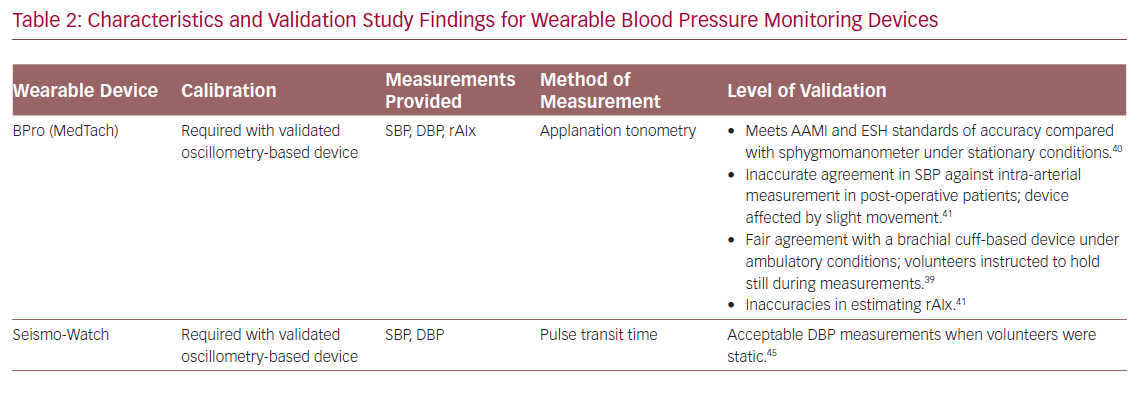
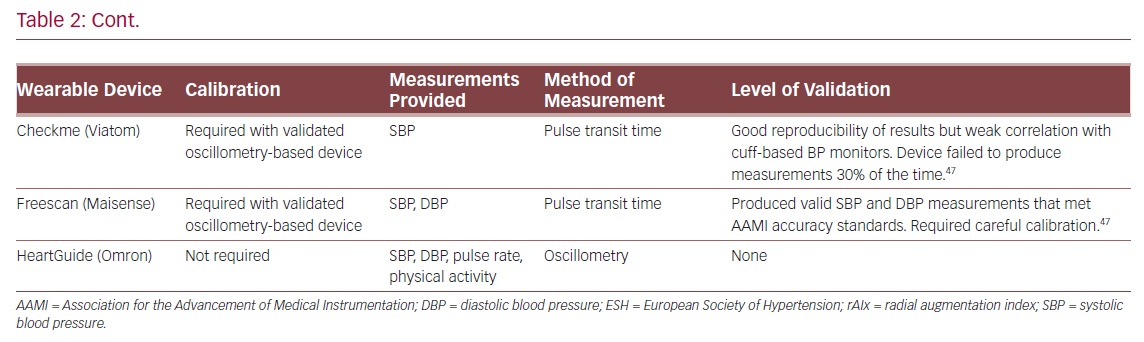
In addition to BP measurement, radial augmentation index (rAIx) can be calculated from radial wave pulse measurements from the BPro.42 The rAIx has been proposed as an estimate of central BP and a useful parameter of vascular function and ageing.43 Although there were discrepancies in rAIx measured by BPro versus a reference device, Vardoulis et al. developed and validated a novel wrist-bound tonometer that produced accurate rAIx readings compared with a reference hand-held tonometer.42,44
The SeismoWatch is an example of a wrist-worn BP monitor that functions via pulse transit time.45 To obtain BP measurements, the watch face contains an accelerometer which is pressed for a short duration on the sternum to obtain a seismocardiogram and determine timing of left ventricular ejection. This is compared with a distal reading from a wrist-obtained photoplethysmogram to obtain the pulse transit time and, therefore, an estimate of BP.45 In a small feasibility study, the device was found to obtain acceptable mean and diastolic BP, but required subjects to perform measurements while static.45 Similarly, Ogink et al. investigated the feasibility of a cuffless BP monitor (Checkme Pro, Viatom) based on pulse transit time to measure systolic BP of hypertensive patients at home.46 The study found that the device produced reproducible BP readings and had good adherence due to its ease of use. However, the device was limited by its inability to measure diastolic BP and failure to measure BP 30% of the time.
Boubouchairopoulou et al. validated a cuffless BP monitor (FreeScan, Maisense) that obtained readings via pulse transit time.47 In large successive feasibility and validation studies (a total of 313 patients were involved in the four feasibility studies), the device was found capable of obtaining systolic and diastolic BP readings when compared with a standard mercury sphygmomanometer. However, accurate readings required a device sensor upgrade and careful initial device calibration in a research setting. Further work is needed to investigate the validity of this prototype device in a clinical setting.
The HeartGuide (Omron Corp) is a novel smartwatch with the ability to monitor BP.48 It uses the traditional oscillometry-based principle where the strap of the wrist-worn device acts as a BP cuff and allows BP measurement for up to 7 days on a single charge. It has additional abilities such as data synchronisation to an online account as well as step count and heart rate monitoring. However, this device is yet to be validated.
Best Medical Therapy Compliance Monitoring
Non-adherence to medication in hypertensive patients is a contributing factor to poor BP control and is associated with higher risks of vascular events, hospitalisation and increased healthcare costs.49 Davidson et al. developed and validated a telemonitoring intervention involving electronic medication trays, Bluetooth-enabled BP monitors and SMS messaging.50 The electronic medication tray (Maya, MedMinder) reminded patients to take their dose at the prescribed time first with a blinking light, then a 30-minute chime and finally an automated phone call or SMS. Patients were reminded to use the Bluetooth-enabled BP monitor (UA-767PlusBT, A&D Engineering) via SMS, and readings were sent from the device to a provided smartphone and a remote data repository via cellular network. If BP measurements were above a predefined threshold, patients were instructed via phone to obtain additional measurements and their physician was alerted. Patients in the telemonitoring group had significant lower systolic and diastolic BP across the study’s duration and a significantly greater proportion achieved BP control.
Frias et al. validated a system involving ingestible sensors and wearable sensor patches (Proteus Discover, Proteus Digital Health) for patients with uncontrolled hypertension and type 2 diabetes.51 The medications of patients in the intervention group were co-encapsulated with ingestible sensors. Once swallowed, the ingestible sensor would activate and send a signal to the adhesive sensor patch. Data from the patch was then transmitted to an online data repository via a smartphone app. Data could be viewed by patients on the mobile app and remotely by their physicians. The mobile app also prompted patients to take their medications at the prescribed times. There were significant improvements in systolic BP and a larger proportion of patients achieved their BP goal in the intervention group. In terms of diabetes, however, there was no significant difference in HbA1c or fasting plasma glucose between intervention and control groups. Physicians with access to the online data made approximately three times more medical decisions per participant and patients with uncontrolled hypertension in the intervention group were more likely to be given a medication adjustment or adherence counselling. However, a comparison of adherence to medication could not be assessed due to the intrinsic design of the study.
Discussion
The NHS Long Term Plan pushes for a digital transformation where digitally enabled care will become mainstream.52 As the NHS shifts towards a digital-first approach for patient consultations, there must be robust and validated methods for remote patient monitoring to aid analysis and decision-making by clinicians. Concurrently, wearable technologies are increasingly ubiquitous, with an estimated 722 million devices connected to the internet.48,49 These trends are set to continue, with shipments of wearable devices expected to almost double from 2019 to 2022, increasing from 226 million to 453.19 million.53 As these technologies become more widespread, clinicians have the responsibility to be early adopters, harnessing the potential they possess especially regarding their telemonitoring properties. However, before these technologies can be implemented clinically, a number of issues must first be addressed.
Compliance and the Digital Divide
The digital divide is the difference between those who possess technological skills and those who do not.54 Cardiovascular diseases most commonly manifest in older patients, the cohort with the lowest rates of smartphone adoption and digital literacy.55 It has been suggested that older adults are reluctant to use mobile electronic devices due to factors such as lack of knowledge, disinterest or vision impairment.56 This potentially limits the use of wearable technology in this population.
Poor adherence has also been demonstrated in other groups. Several studies included in this review mentioned limitations of technology due to know-how or non-use. Ogink et al. found that only 36% of participants correctly performed BP measurements with their cuffless monitor after instruction.46 However, the authors suggested that education using a set procedure or an instructional video may increase correct usage.46,57,58 Nicolaï et al. found that 30% of patients in the wearable intervention group did not use the prescribed fitness tracker for the duration of the study or at all.33 Similarly, Endicott et al. reported 43% non-use of fitness trackers by participants.25 Although adherence to the use of fitness trackers was very good (>80%) in a series of trials by Gardner et al., it was noted that participants were volunteers.31,32 This exposes the study to selection bias, where patients who were more comfortable with technology or were more motivated were more likely to participate.
Further work is needed to determine digital literacy in vascular patients. This could be achieved through validated literacy questionnaires such as eHEALS for eHealth literacy or MDPQ-16 for mobile device proficiency.59,60 Thus, appropriate wearable and telemonitoring interventions may be used and adequate coaching given to participants.
Digital Infrastructure
The expansion of wearable and telemonitoring interventions is limited by infrastructure. Hovey et al. investigated several practical aspects that negatively affected telemonitoring.61 Suboptimal internet connectivity, especially in rural areas, affected data collection and patient adherence. There were hardware issues that needed to be diagnosed remotely and required replacement equipment, thereby reducing patient access and increasing costs. Software issues were identified that could affect large-scale implementations of telemonitoring. For successful wearable and telemonitoring interventions, there is a need for robust and reliable infrastructure. Larger-scale extended studies could be conducted to validate the large-scale extension of a wearable or telemonitoring intervention.
Cost
The up-front cost of novel technology is often cited as prohibitive to widespread adoption. In fact, wearable and telemonitoring interventions may have cost benefits compared with conventional management. With regards to PAD management, a standard 3-month supervised exercise programme was estimated to cost about £235–£345 per patient in a 2013 study.62 In comparison, fitness trackers can cost as little as £21 per unit, although this can be considerably higher with more advanced devices. Furthermore, this cost can be offset by the reduction in travel expenses for the patient. These interventions additionally allow the individual tailoring of exercise requirements for each patient, which cannot be achieved with group supervised exercise.
Similarly, telemonitoring hypertension interventions may be cost-efficient. Low adherence to antihypertensive therapy was estimated to cost US$3,574 per patient over a 3-year period.49 In a 2015 study, Davidson et al. estimated the cost of their telemonitoring intervention (electronic medication tray, SMS encouragement, Bluetooth BP monitor and associated support staff) to be US$65 per month for patients who owned a smartphone and US$128 per month for those who did not.50 Investigators noted that the average cost of an emergency department visit to be US$5,923 and the intervention group had a 57% reduction in visits compared with those receiving standard care, therefore giving a US$17,548 cost saving over a 6-month period. In addition, as telemonitoring can potentially identify patients with white-coat hypertension. It may avoid overtreatment and associated medication costs.7 There is a need, however, for further work to formally evaluate cost-effectiveness of wearable and telemonitoring interventions in cardiovascular patients.
Conclusion
Wearable technologies are becoming increasingly prevalent and there is some evidence that wearable and telemonitoring interventions may be beneficial for managing vascular patients and keeping them out of hospital. With healthcare moving towards a digital future, it is inevitable that wearable devices and telemonitoring will become increasingly widespread in the clinical environment. More work is needed to validate these technologies with regards to digital literacy of patients, cost-effectiveness and supporting digital infrastructure before widespread implementation.