Peripheral artery disease (PAD) affects more than 200 million people worldwide, and its prevalence is increasing due to numerous risk factors, including age and diabetes.1–3 Endovascular treatments, primarily percutaneous transluminal angioplasty (PTA), have become common first-line therapy for symptomatic PAD.4,5 However, mechanical dilatation of the vessel during PTA often results in vessel dissection.6–8 Left untreated, lesions with dissections have high 1-year restenosis rates of 40–60% and a threefold increase in 6-month target lesion revascularisation (TLR) compared to lesions without dissections.7,9–12 The most commonly employed treatment for dissections is stent placement. Stents improve procedural success and patency relative to PTA. However, stents exert a strong radial force on the vessel wall and the extensive nitinol surface area has the potential to promote inflammation, hyperplasia and in-stent restenosis at not insignificant rates.13–16
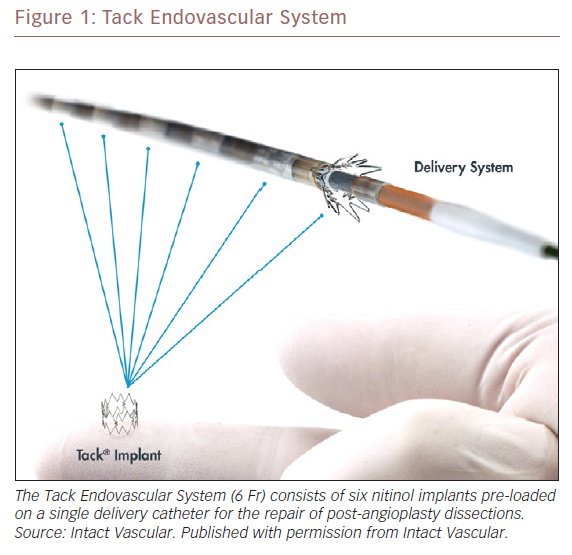
Tack Endovascular System
The sub-optimal outcomes associated with the current treatments for dissection led to the development of the Tack Endovascular System (Intact Vascular). Tack implants are designed for focal dissection treatment. Tacks are shorter than stents (6 mm) and constructed with an open cell design, which limits the metal surface in contact with the luminal wall and exerts a lower chronic outward force when compared to stents. The Tack Endovascular System consists of a 6 Fr (2.0 mm) delivery catheter pre-loaded with six independent nitinol implants 6 mm in length (Figure 1). The implants are of a single-size and self-expanding and can treat a range of vessel diameters from 3.5–6.0 mm.
The recently published single-arm Tack Optimized Balloon Angioplasty II (TOBA II) study evaluated 213 patients who developed dissections following PTA of the superficial femoral arteries (SFA) or proximal popliteal arteries (PPA) and were treated with Tacks. The primary patency and freedom from clinically driven TLR at 1 year were 79.3% and 86.5%, respectively. Clinically driven target lesion restenosis (CD-TLR) occurred in 31 (14.6%) of patients in the first year post-index procedure.17
The incidence rates and extent of restenosis can vary due to the vessels treated, the treatment modality utilised and patient comorbidities.18 To better understand the severity and extent of restenosis in peripheral vessels and the effect on these patterns on successful treatment, Tosaka et al. evaluated 116 patients with in-stent restenosis (ISR) and proposed a three-level classification of severity, ranging from class I (focal lesions) to class III (total occlusion).19
Aim
The objective of this investigation was to evaluate the restenosis patterns in patients who received Tacks as part of the TOBA II study and compare these results to the lesion characteristics published for nitinol stent implantation. Furthermore, the pattern of restenosis relative to the placement of Tack(s) was evaluated.
Methods
The TOBA II study was a prospective, single-arm, multicentre clinical investigation to evaluate the safety and efficacy of the Tack Endovascular System for the repair of all post-PTA dissections (NCT02522884). It was conducted in compliance with the International Conference on Harmonization Good Clinical Practice, ISO 14155 and the Declaration of Helsinki and the ethics committees at the participating sites approved the study protocol. Study participants provided written informed consent before undergoing any study procedures.
Patients included in this study were required to meet the following major inclusion criteria: Rutherford Category 2–4 claudication; atherosclerotic lesions (≥70% diameter stenosis) in the SFA, PPA or both; and lesion length ≥20 mm and ≤150 mm for lesions with 70–99% stenosis and ≤100 mm for occluded arteries.
Patients were treated with balloon angioplasty or Lutonix (BD) drug-coated balloons, based on physician preference. Post-angioplasty, lesions with <30% residual diameter stenosis and at least one dissection of any severity were treated with the Tack Endovascular System. To treat the post-PTA dissections, Tacks were deployed singly or in multiples at the discretion of the treating physician.
Angiographic evaluation of the index target lesions in TOBA II was conducted by an independent core laboratory (Yale Cardiovascular Research Group Angiographic Core Laboratory, New Haven, CT, US). This evaluated the per cent diameter stenosis, lesion length and degree of calcification of the index lesion. The core laboratory also provided post-treatment grades of dissections, using the National Heart Lung and Blood Institute classification system.20 For patients who had target lesion restenosis in the first year following the index procedure, the angiographic images were analysed and scored by an independent core laboratory (Syntropic CoreLab, Columbus, OH, US).
The restenotic lesions were evaluated for per cent diameter occlusion and lesion length. They were classified using the methodology of Tosaka et al. as follows: class I – focal (<50 mm in length) lesions located within the stent body, at the stent edge, or a combination of both; class II – diffuse (>50 mm in length) including both stent body and stent edge lesions; and class III – total occlusion. As noted, the Tosaka classification system was developed to describe lesions in full-length stents. Unlike stents, Tacks, by design, do not cover the full length of treated lesions and multiple Tacks can be used to treat dissections. Due to this unique feature, the core lab also provided an analysis of the location of target lesion restenosis relative to Tack location(s) using the following qualitative analysis:
- proximal – lesion located proximal to an area that was Tacked;
- at – lesion located within a Tack;
- distal – lesion located distal to a Tacked area;
- between – lesion located between Tacks; and
- involving multiple – lesions located at multiple Tacks.
Statistical Analysis
Continuous and categorical variables are expressed as mean ± SD and number (per cent of total), respectively. Analysis of variance was utilised to evaluate continuous variables. Fisher’s exact test was used to evaluate categorical variables. Unpaired t-tests were used to compare continuous variables between the Tosaka et al. and TOBA II cohorts. A p value of <0.05 was considered as statistically significant.
Results
A total of 213 patients were enrolled in the TOBA II study, of whom 31 (14.6%) required a clinically driven revascularisation within the first year of the index procedure. Of these 31 patients, 28 had angiograms that were evaluable by the core laboratory and 14 (45.2%), five (16.1%) and nine (29%) were graded as having Tosaka class I, II and III type lesions, respectively.
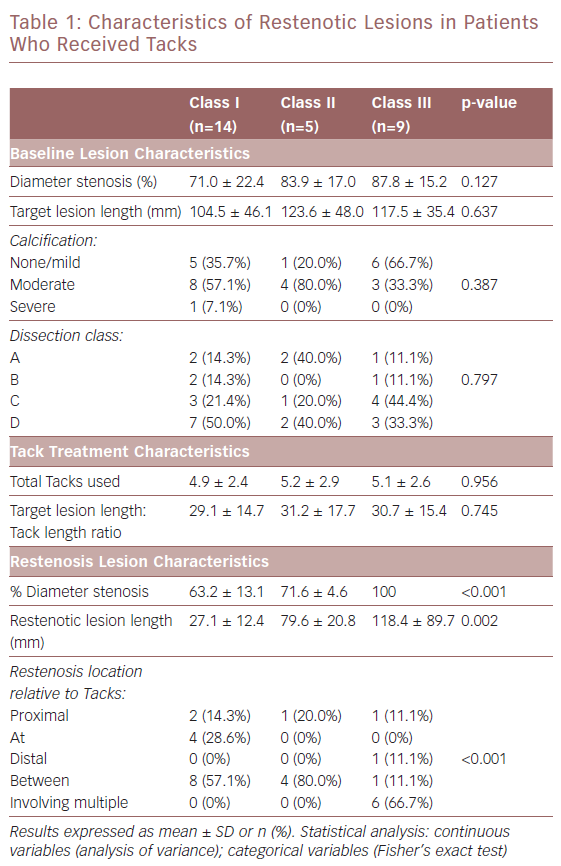
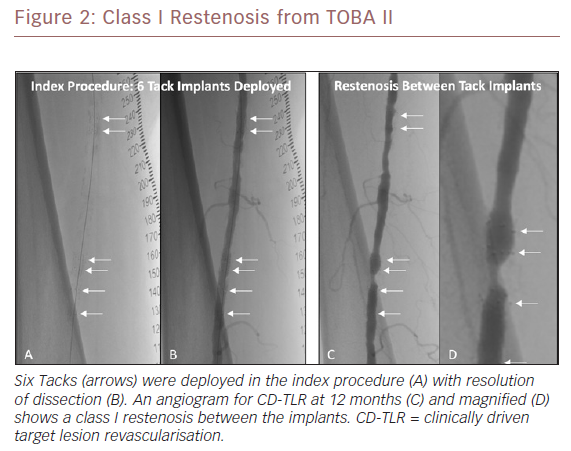
Table 1 summarises the baseline lesion, Tack treatment and restenosis lesion characteristics by lesion classification. There were no significant differences (p>0.05) in lesion length, degree of calcification, or dissection class between the three groups. There was no significant difference in the total number of Tacks used to treat dissections, which were 4.9 ± 2.4, 5.2 ± 2.9 and 5.1 ± 2.6 in class I, II and III lesions, respectively. Similarly, there was no significant difference in the target lesion length:Tack length ratio. Evaluation of the restenotic lesions showed a significant difference (p=0.002) in lesion length between the restenosis classes: 27.1 ± 12.4 mm (class I); 79.6 ± 20.8 (class II); and 118.4 ± 89.7 mm (class III). Location of lesions relative to location of the Tack(s) was also significant. Across all three restenosis classifications, 18 patients (64.3%) had lesions that did not involve the Tacks (proximal, distal and between categories). Of note, 66.7% of the lesions with total occlusions (class III) involved multiple Tacks, while none of the class I or II lesions involved multiple Tacks. Figure 2 illustrates a class I restenosis from the TOBA II study.
Comparison of TOBA II and Tosaka Cohorts
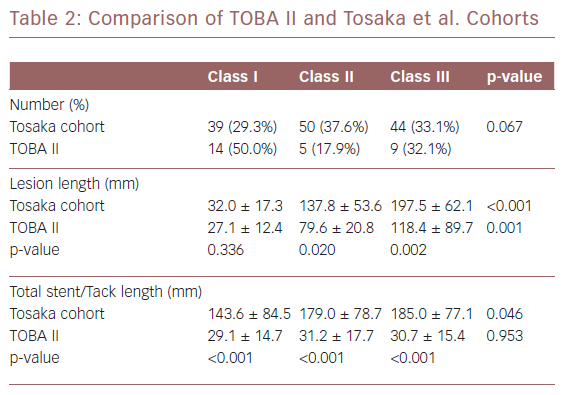
The Tosaka et al. and TOBA II cohorts are compared in Table 2. The Tosaka et al. study was a retrospective analysis of 133 limbs in 116 ptients with ISR.19 In comparing the two studies, the percentage of patients with each of the three restenosis classifications was not significantly different (p=0.067). There was a trend toward more limbs with class I restenosis (the least clinically severe category) in the TOBA II cohort (50.0% versus 29.3%).
The class I mean lesion length was not significantly different (p=0.336) between the two cohorts. However, the class II and III mean lesion lengths in the stented cohort were significantly (p=0.020 and p<0.001, respectively) longer compared to the length of restenosis in the TOBA II cohort. Stent length was increased with severity of restenosis and the difference across the restenosis classifications was significantly (p=0.046) longer. In comparison, there was no significant difference (p=0.953) in total Tack length across the three restenosis classifications. Across all Tosaka classes, the total Tack length was significantly (p<0.001) shorter than the total stent length.
Discussion
Tacks were developed to provide a minimal metal alternative to stents for the treatment of post-PTA dissections. Like stents, Tacks facilitate the apposition of dissection flaps to the luminal surface. However, they have a reduced surface area when compared to stents. Increased surface area and the more expansive metal scaffold of stents promote the development of neointimal hyperplasia and predispose to stent fracture, both of which can facilitate the development of restenosis in a target lesion.21–23 Histologically, Tacks have been shown to have a reduced hyperplastic response when compared to stents.24 The TOBA II clinical study, which evaluated Tacks for treatment of dissections following treatment of femoropopliteal disease, met its 1-year primary endpoints of primary patency (79.3%) and freedom from clinically driven TLR (86.5%). The 1-year restenosis rate in this cohort (14.6%) is favourable when compared to stents.17
A primary motivation for developing lesion scoring systems was to better understand the prognostic significance of angiographic patterns of restenosis to the outcomes following secondary revascularisation procedures.
Tosaka et al. identified nearly equal numbers of class I, II and III lesions (29.3%, 37.6% and 33.1%) in their cohort. Of note, a single centre retrospective analysis of ISR by Armstrong et al. showed nearly similar proportions of ISR, with 37.3%, 29.3% and 33.3% in class I, II and III, respectively.25 The TOBA II cohort had a higher percentage (50.0%) of patients with less severe class I lesions than these two studies, but the percentage with class III lesions was 32.1%, similar to both Tosaka et al. and Armstrong et al.
As in the Tosaka et al. analysis, there are key differences between class I/II and class III lesions. Class I/II lesions had nearly identical 3-year recurrent ISR rates of 49.9% and 53.3%, respectively, which was significantly better than class III lesions (84.8%). During 2 years of follow-up Armstrong et al. showed significant (p=0.04) differences in the rates of repeat restenosis: 39% class I; 67% class II; and 72% class III.25
A key and statistically significant difference (p<0.001) between the TOBA II cohort and the Tosaka cohorts is the shorter lesion length of the class II and III lesions in TOBA II patients. The Armstrong study displayed similar lesion lengths to the Tosaka study. Lesion length was identified as a significant univariate predictor of recurrent ISR.
Tacks allow for multiple potential options due to their minimalist approach of reduced metal burden compared with stents. These options could include repeat angioplasty, drug-coated balloon angioplasty, laser atherectomy, or other modalities. Future studies should investigate the optimal treatment strategies for in-Tack restenosis.
One additional analysis that was unique to TOBA II was the localisation of restenosis relative to Tack placement. These data showed that only four out of 19 (21%) of class I/II lesions were within the body of the Tack. In class III lesions, 66.7% involved multiple Tacks. As with stent-treated lesions, class III lesions with Tacks were the longest lesion type. Unlike class III stent lesions where the restenosis essentially covered the majority of stent surface, Tacks only represented about 25% of the total lesion length in class III Tack lesions, which may be indicative of broad diffuse disease rather than the presence of Tacks. Overall, the 14.6% restenosis rate in the TOBA II cohort is lower than the rate of restenosis than the 20–37% range reported for stents and is within the 3–20% range reported for non-stent technologies.13–16,18
There are a few limitations to this study. Although the sample size of the TOBA II cohort is small, it is still the largest cohort to date in which patients received standard treatment. It is important to note that the Tosaka classification was developed to describe re-stenosis in long stents. However, it is widely accepted longer lesions and occlusions are associated with inferior secondary patency rates compared to less severe lesions. As such, it is worth comparing the patterns of re-stenosis between these two groups. Conclusions on comparative prognostic data on recurrent re-stenosis rates between the two groups can be achieved only with an appropriately designed randomised controlled trial.
Conclusion
This the first core lab-adjudicated observational data report on patterns of in-Tack stenosis. These data show that Tacks have a relatively low rate of target lesion restenosis at 1 year. Furthermore, Tack restenotic lesion analysis demonstrate a prevalence of both class I and shorter lesions.