Thoracoabdominal aorta occupy an inaccessible location high in the retroperitoneum, and their branches are the sole source of blood to all the abdominal organs. Both features complicate conventional surgical repair, which requires wide aortic exposure and prolonged interruption of aortic flow. Since thoracoabdominal aortic aneurysm (TAAA) is a disease of elderly male smokers, many patients have serious cardiac and pulmonary co-morbidities. In addition, many have already undergone open abdominal aortic aneurysm repair. These patients tolerate the stresses of conventional open repair poorly.1-4 The reported morbidity and mortality rates of conventional surgical repair of TAA vary widely, depending on the type of disease (dissection versus aneurysm), the extent of disease,3,4 and the expertise of surgeons, anaesthesiologists and cardiologists.2 US national and statewide audits provide the most representative picture of current results. In California, for example, the 30 day and one year mortality rates are 19% and 31%, respectively. Paraplegia rates are similarly alarming.1
In theory, an endovascular approach to TAAA repair has two main advantages. First, the stent-graft is inserted through a trans-luminal route; there is no need to expose the aneurysm. Second, the grafts are inserted without clamping the aorta; there is no need to interrupt flow to the abdominal organs. More than a decade has passed since endovascular repair of abdominal aortic aneurysm (AAA) and thoracic aortic aneurysm (TAA) first started to displace open surgery as first-line therapy, yet endovascular repair of TAAA is still confined to a handful of centres worldwide.
The fundamental problem is how to maintain flow to the visceral branches of a TAAA while excluding the aneurysm itself from the circulation. The two main alternatives are: a combination of visceral artery bypass and endovascular exclusion, and branched stent-graft insertion.
The first option is a combination of conventional visceral bypass and endovascular aneurysm exclusion. The surgical bypass grafts to the visceral arteries originate outside the field of endovascular exclusion, usually from the distal aorta or common iliac aneurysm. Once the aneurysm has been 'debranched', endovascular exclusion can proceed in the usual way.
The second option, multi-branched stent-graft implantation, eliminates the need for trans-cavitary incisions and interruption of visceral arterial flow. However, the procedure is far from simple, because each branch of the stent-graft represents a separate line of insertion. All these lines of insertion intersect in the aorta where the branches join the trunk.
The nature of that junction determines the type of branched stent-graft. Unibody multi-branched stent-grafts have permanent sutured connections, whereas modular fenestrated multi-branched stent-grafts have only a ring of contact between each balloon expanded covered stent and the corresponding hole (fenestration) in the wall of the primary stent-graft. Modular cuffed multi-branched stent-grafts occupy an intermediate position between these two extremes. The primary stent-graft of a cuffed multi-branched stent-graft has branches sewn into its surface, but these are not long enough to reach the orifices of the visceral arteries. Instead, they serve only to enhance the connection between the primary stent-graft and self expanding covered stents.
Relative Merits of Different Approaches
Visceral artery bypass alone would be a big operation for the typical high-risk patient with TAAA. Subsequent stent-graft implantation may not add much in the way of dissection, but the constrast load is nephrotoxic, the sheath valves leak blood with every catheter exchange, and the additional operative time means additional anaesthesia. The combination of visceral bypass and endovascular aneurysm exclusion could hardly be described as a minimally invasive procedure. Nevertheless, recent series have reported low mortality and morbidity rates in high-risk patients with extensive aneurysms.5,6 The low rate of paraplegia is particularly noteworthy, because none of these cases included intercostal artery re-implantation, and most required near total exclusion of all the native aorta, from the subclavian origin to the bifurcation. It appears that haemodynamic stability is more important than intercostal preservation in preventing paraplegia.7
In theory, the entirely endovascular operation of multi-branched stent-graft implantation should be even less invasive than the bypass/exclusion. In practice, the technical challenges inherent in multi-branched stent-graft implantation introduce a wide range of potential complications. In this regard, simpler is better. The irreducible complexity of the unibody multi-branched stent-graft increases exponentially with every additional branch.8,9 The stent-graft and all the branches are delivered as a single unit. If any part fails, the whole device fails. The modular approach, on the other hand, breaks the procedure down into a series of bite-sized parts. Not only are modular stent-grafts simpler, they are more versatile. Variations in component selection and intercomponent overlap can accommodate intra-operative findings.
The sole disadvantage of the modular approach is the potential for component separation, especially when the inter-component junction is represented by a single ring of contact between the margin of a fenestration and balloon expanded covered stent.10,11 Fenestrated modular stent-grafts are not stable enough for use in TAAA where the covered stent has to bridge a wide gap between the primary stent-graft and the wall of the aneurysm. The 15-20 mm overlap provided by a cuff is far superior in this regard. Cuffed stent-grafts are also easier to plan, easier to make and easier to implant,12 because they do not require the same high degree of precision as fenestrated stent-grafts. Nevertheless, all devices of this type are still individually made in Australia. The delay from sizing to implantation is currently four to eight weeks.
Cuffed Multi-branched Stent-grafts
Stent-graft Design
All modular fenestrated and multi-branched stent-grafts employ the basic Zenith design, with stainless steel Z-stents sutured to surgical woven polyester. The proximal stent has many barbs to enhance attachment. Three trigger wires control deployment. A constraining wire keeps the unsheathed stent-graft in a partially expanded state. Two other wires secure the proximal and distal ends of the stent-graft to the shaft of the delivery system. Sheath sizes range from 20 to 24 French. Extensive aneurysms often require additional aortic stent-grafts above or below the cuffed component at the visceral artery level. This is typically wider at the ends than in the middle. The narrow central segment carries the cuffs. The cuffs are little barrels of woven polyester, 15-18 mm in length and 6-8 mm in diameter, supported by Nitinol wire loops. They are sutured into the wall of the primary stent-graft with one end opening to the inside and one end open to the outside. Most cuffs, like most visceral arteries, are downgoing, with the proximal end opening to the inside of the main stent-graft and the distal end opening to the outside. Depending on specific anatomic requirements, they can also be upgoing, externally mounted, or even helical.
Stent-graft Implantation
The author places a spinal catheter for cerebrospinal fluid (CSF) pressure monitoring and drainage. In most cases, the spinal catheter also serves as a means of regional anaesthesia. Some patients with large stent-grafts and small arteries require surgically created conduits to the common iliac artery, but most are amenable to trans-femoral insertion. A brite-tip selective catheter in a visceral artery provides a reference point to guide stent-graft position with the usual downgoing cuffs 10-25 mm above the corresponding arterial orifices.
The author inserts the self-expanding covered stents through a brachial artery, usually the left. The route from there to the visceral aorta can be very tortuous. Insertion of catheters and delivery systems through a conduit of co-axial, kink resistent sheaths (Flexor, Cook, Bloomington, IN) minimises the potential for redundant loop formation (in the arch). The outer 12 French sheath provides support, and protects the aorta. The inner 10 French sheath is large enough to allow contrast injections around a Fluency covered stent delivery system.
All covered stent insertions follow the same sequence: cuff catheterisation, sheath advancement into the aneurysm, angiographic localisation of the target artery, catheterisation of the target artery, selective angiography, stiff guidewire (Rosen, Cook, Bloomington, IN) insertion, stent-graft insertion, and Wallstent insertion. Most covered stents measure 60 mm in length, and 7-9 mm in diameter. The Wallstent prevents kinking and helps stabilise covered stent position.
Post-operative Management
Multi-branched cases stay in the intensive care unit at least two days after the operation, for CSF and blood pressure monitoring. In the absence of lower extremity neurological symptoms, the CSF drain, arterial line and Foley catheter are all removed on the second post-operative day. All patients resume normal diet, and receive loading doses of Plavix on the first post-operative day .
Outcome
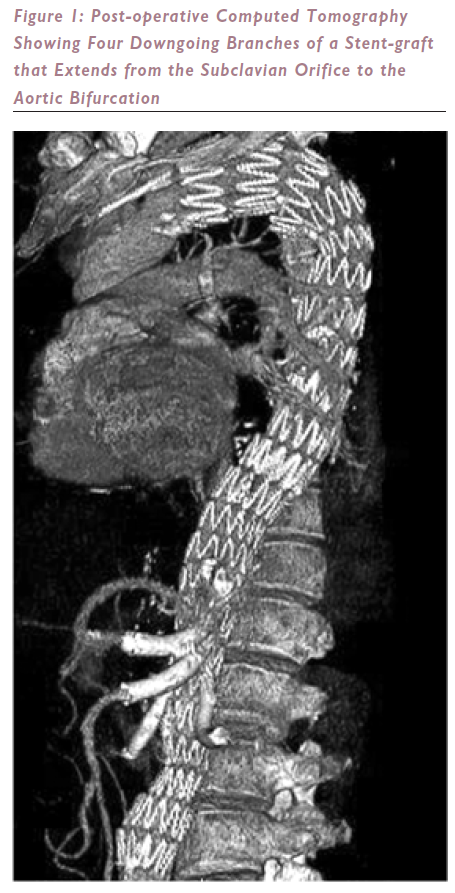
In the past year the author has inserted 16 cuffed multi-banched stent-grafts bearing a total of 60 branches, only one of which has occluded. All the other branches remain patent. Eight of these stent-grafts covered the aorta, from subclavian orifice to bifurcation (see Figure 1). The only case of paraplegia occurred in the setting of hypotension and renal failure caused by a wire-induced perforation of a branch of the sole renal artery. This patient refused dialysis and died. There have been no other deaths, and no other cases of paraplegia, although one patient was transiently (less than five minutes) paraparetic during an episode of relative hypotension (110 mmHg systolic) on the first postoperative night.
Current and Future Roles of Different Endovascular Stent-grafts in the Management of Aortic Aneurysm
At UCSF modular cuffed multi-branched stent-grafts are used for elective endovascular repair of TAAA in high-risk patients, and reserve combined visceral bypass/endovascular repair for more urgent repair of very large or symptomatic aneurysms and healthier patients. Simple fenestrations are used for juxtarenal aneurysms, and branched fenestrations for pararenal aneurysms. The author has also tried to use a combination of fenestrations and cuffs in the same stent-graft, in cases where not all of the visceral segment was dilated to the same degree. However, this was found difficult to do, because cuffs and fenestrations cannot be used in close proximity. The cuffed approach requires space between the stent-graft and the aorta, whereas the fenestrated approach requires direct contact between the stent-graft and the aorta.
Despite regulatory hurdles that block access to the necessary devices and impede dissemination of the necessary skills, the author expects that modular cuffed multi-branched stent-grafts will probably become the mainstay of treatment in most cases of pararenal and thoracoabdominal aortic aneurysm. The author also anticipates that covered stents will largely replace the uncovered stents currently used to keep simple fenestrations in place, because the potential for leakage is less.