Saphenous vein grafts (SVGs) are commonly used during coronary artery bypass graft surgery (CABG) for severe coronary artery disease. However, SVGs are prone to both degeneration and occlusion, leading to poor long-term patency compared with arterial grafts. Previous reports suggest rates of SVG failure in the first 12–18 months may be as high as 25 %.1–4 SVG neointimal hyperplasia and accelerated atherosclerosis diminish the long-term benefits of CABG, while subsequent SVG interventions are plagued by plaque embolisation and no-reflow phenomenon. Percutaneous coronary intervention (PCI) of SVGs is associated with worse clinical outcomes compared with native coronary artery PCI,5,6 but certain strategies may help mitigate complications. In this review, we discuss risk factors for SVG intervention and the optimal approaches for treating this challenging subset of patients.
Pathophysiology of Saphenous Vein Graft Complications
Various factors contribute to SVG deterioration and occlusion, which may ultimately require revascularisation. Approximately 10–15 % of SVGs occlude within 1 year and 50 % fail by 10 years.7 Platelet aggregation, growth factor secretion, endothelial dysfunction, inflammation, luminal foam cell accumulation, decreased local fibrinolytic potential from plasminogen activator inhibitor-1 upregulation and marked intimal hyperplasia contribute to SVG occlusion within the first 12–18 months post-CABG.8–14 Occlusions after 12–18 months occur following lipid deposition within intimal hyperplasia, eventually forming atherosclerotic plaque.14 Increased pressure load from vein graft arterialisation induces this development of neointimal growth and atherosclerosis.7 Deteriorating SVG lesions also possess thinner, more friable fibrous caps compared with native coronary artery lesions.15,16 this increases the incidence of plaque embolisation and platelet aggregation,17,18 especially during SVG interventions.
Atheroembolic debris liberated during SVG intervention becomes lodged in distal capillaries, while the release of neurohormonal factors such as serotonin can induce vasospasm. Slow or no-reflow phenomena may subsequently follow, which are associated with both periprocedural angina and ischaemic ST-segment changes.19 The exact mechanism of no-reflow remains unclear, but it has been hypothesised that endothelial swelling, neutrophil infiltration and platelet aggregation induce microvasculature spasm and obstruction.20,21 SVG intervention is thus associated with higher rates of in-stent restenosis, target vessel revascularisation (TVR), periprocedural MI and in-hospital mortality compared with PCI for native coronary circulation. The severity of these potential consequences makes proper patient selection and optimal technique essential during invasive SVG revascularisation.
Predictors of Adverse Outcomes
The strongest predictors of SVG intervention 30-day major adverse cardiac events (MACE) are angiographic estimations of SVG degeneration and plaque volume.22,23 Another study analysing patients undergoing SVG intervention with distal embolic protection reported that lesion length has the strongest correlation with short-term adverse events.24 A graded increase in MACE was observed with increasing lesion lengths, perhaps correlating to the increase in SVG plaque burden.
The data on the impact of gender have provided mixed results. One study suggested that male patients were more inclined to have worse outcomes,25 but another study reported that female patients had a higher 30-day cumulative mortality rate (4.4 % versus 1.9 %, P=0.02).26 Female patients also had significantly higher rates of vascular complications (12 % versus 7.3 %; P=0.006) and post-procedural acute renal failure (8.1 % versus 4 %; P=0.02) compared with male patients.
Chronic renal insufficiency (serum creatinine ≥1.5 mg/dl) was a significant predictor of 1-year MACE in patients who underwent SVG intervention with drug-eluting stents (DES) (hazard ratio [HR] 2.2; 95 % CI [1.1–4.3]; P=0.03).27 There was also a trend toward higher rates of TVR in the renal insufficiency group (21.8 % versus 10.3 %; HR 2.42; 95 % CI [0.94–6.24]; P=0.059). Another study reported that patients with renal insufficiency had higher mortality rates following SVG PCI.28
Patients commonly experienced elevations in levels of creatine kinasemyocardial band (CK-MB) following SVG intervention.17 Approximately 15 % of patients who underwent SVG intervention were found to have CK-MB levels >5x the upper limit of normal (ULN), which increased the 1-year mortality rate in patients with normal CK-MB from 4.8 % to 11.7 % (P<0.05 analysis of variance [ANOVA]). Even minor elevations in CK-MB levels (>1x to <5x ULN) were associated with an increased 1-year mortality rate (6.5 %; P<0.05 ANOVA).17
Lesion Evaluation and Patient Selection
Lesion Evaluation
The decision to perform SVG revascularisation should predominantly be based on patient symptoms and evidence of myocardial ischaemia in regions supplied by the SVG.
Fractional flow reserve (FFR) is used to determine the significance of native coronary vessel stenosis, but has not been well studied in SVG lesions. Limited studies show that FFR has low sensitivity, but an acceptable specificity and negative predictive value compared with stress myocardial perfusion imaging in assessing the significance of SVG lesions.29 Myocardial perfusion imaging has good specificity for detecting ischaemia after CABG, but variable sensitivity in detecting angiographically significant graft stenosis.30
Intravascular ultrasound (IVUS) may play a role in SVG disease evaluation, as positive remodelling on IVUS is a strong predictor of post-intervention no-reflow.31 However, IVUS has not been adequately evaluated in prospective SVG intervention trials to support intervention based on IVUS findings alone.
Multidetector computed tomography (MDCT) provides adequate visualisation of SVGs given their reduced motion and large lumens.32 Although it provides a sensitivity of 96 % and a specificity of 95 % in evaluating graft patency,33 it is limited in its ability to visualise distal anastomosis sites. Further advancements in this technique are needed to match the gold standard of coronary angiography.
Patient Selection
Prophylactic stenting of intermediate SVG lesions has been advocated given that the progression of SVG disease can be rapid. In the Moderate Vein Graft Lesion Stenting With the Taxus Stent and Intravascular Ultrasound (VELETI) trial, 57 patients with moderate (30–60 %) SVG stenosis were randomised to medical therapy alone or revascularisation with DES.34 Both minimal luminal diameter and percent stenosis were decreased in the DES group. The MACE rates at 1 and 3 years were lower in the DES group compared with the medical therapy group (At 1 year: 3 % versus 19 %, P=0.09; at 3 years: 3 % versus 26 %, P=0.02).35,36 The VELETI trial was underpowered for clinical endpoints. The larger 450-patient VELETI II trial randomised patients with intermediate SVG lesions to either SVG intervention with paclitaxeleluting stents or medical therapy alone.37 This study was terminated prematurely due to slow patient enrolment.
Percutaneous revascularisation is not recommended in patients with chronic total SVG occlusion. A study of 34 patients with chronic total SVG occlusions reported that successful recanalisation with stent implantation was low (68 %).36 Rates of TVR and in-stent restenosis at 18-month follow-up were very high (61 % and 68 %, respectively) in patients who underwent successful stenting.
Intervention Technique
Preparation
Given inferior long-term outcomes of SVG intervention compared with native vessel PCI,38,39 revascularisation of the bypassed native vessel should be considered only if the indication is clear. Knowledge of the CABG operative report details, including graft locations, number of grafts and complications encountered during the surgery, is helpful. Previous angiography can provide additional benefit in guiding angiography and revascularisation.
Optimal guiding-catheter support is imperative to the procedural success of SVG intervention. The size of the aorta combined with the position and angle of the SVG determines the type and size of catheter best suited for engagement. The multipurpose catheter is often used for right coronary graft interventions, especially if the graft take-off is steep and inferior. The Judkins right (JR) catheter or Amplatz left (AL) catheter may be used if the angle of the origin of the SVG to the right coronary artery is more horizontal. The JR catheter can engage left coronary artery grafts as well, especially those with a horizontal takeoff from the aorta. Other available catheters include the AL, left bypass and hockey stick catheters.
Pre-dilation Versus Direct Stenting
Although pre-dilation with balloon angioplasty is often employed in native vessel PCI to optimise lesions, this strategy may not be suitable for SVG interventions. Direct stenting provides the potential benefit of trapping debris and decreasing distal embolisation that can occur with pre-dilatation. Patients who underwent direct stenting were associated with nearly a 50 % reduction in CK-MB level elevations >4x normal (13.6 % versus 23 %; P<0.12), overall lower maximum CK-MB release (9.5 versus 19.6 IU/L; P<0.001) and reduction in non-Q-wave MI (10.7 % versus 18.4 %; P<0.02) compared with angioplasty first without distal protection.40 However, one retrospective study of patients undergoing direct stenting without distal protection versus angioplasty followed by stenting with distal protection reported that the rate of increase in CK-MB levels >2x ULN and rates of MACE were no different in-hospital or at 30 days.41 Prospective randomised trials are needed to confirm whether direct stenting versus pre-dilation is most effective in reducing distal embolisation.
Stent Selection
Stent Sizing
Proper stent sizing is crucial to ensuring long-term stent longevity and vessel patency. One study explored the concept of undersized stenting to reduce distal embolisation. Hong et al. analysed outcomes of SVG intervention with DES in three groups according to the ratio of stent diameter to average IVUS reference lumen diameter (group I: <0.89 mm, group II: 0.9 to 1.0 mm, group III: >1.0 mm).42 Incidence of CK-MB level elevation >3x normal was 6 %, 9 % and 19 %, respectively (P=0.03) without an increase in clinical events at 1 year. The hypothesised reduction in distal embolisation and periprocedural MI with undersized stenting must be weighed against the conceivable risk of increased stent restenosis and thrombosis.
Bare-metal Stents
The Saphenous Vein De Novo (SAVED) trial reported that bare-metal stents (BMS) were associated with higher procedural success rates compared with balloon angioplasty (47 % versus 36 %; P=0.11) with a lower MACE rate throughout 240 days (26 % versus 38 %; P=0.04).43
Covered Stents
Covered stents comprised of polytetrafluoroethylene (PTFE) membranes were developed to act as local filters that would trap plaque debris extruding between stent struts. A multicentre registry suggested favourable results with their use in SVG intervention.44 However, randomised trials failed to show superiority over BMS.45–48 The Stents in Grafts (STING) trial reported no differences in MI, target lesion revascularization (TLR) or death with PTFE-covered stents compared with conventional stents.45 The Randomized Evaluation of Polytetrafluoroethylene Covered Stent in Saphenous Vein Grafts (RECOVERS) trial also reported similar clinical outcomes between PTFE-covered stents and BMS with higher rates of non-fatal MI with PTFE-covered stents.46 These findings were confirmed in the Symbiot III trial, which found PTFE-covered stents to have similar clinical outcomes and rates of restenosis as BMS.47 Finally, the Barrier Approach to Restenosis: Restrict Intima to Curtail Adverse Events (BARRICADE) trial reported more long-term target vessel failure in patients with covered stents compared with BMS.48
There remain two covered stents that show potential benefit in treating degenerated SVGs, although they lack long-term head-to-head comparison data with BMS. The SESAME first-in-human trial reported that patients treated with a nanosynthesised, membrane-covered self-expanding, super-elastic, all-metal endoprosthesis stent (SESAME stent, Advanced Bioprosthetic Surfaces Ltd) had 0 % 30-day and 14 % 9-month MACE rates.49 The other stent under study is the MGuard stent (InspireMD), with preliminary evaluation in one study demonstrating an excellent performance with no angiographic/procedural complications or adverse events in 30-day follow-up.50
Drug-eluting Stents
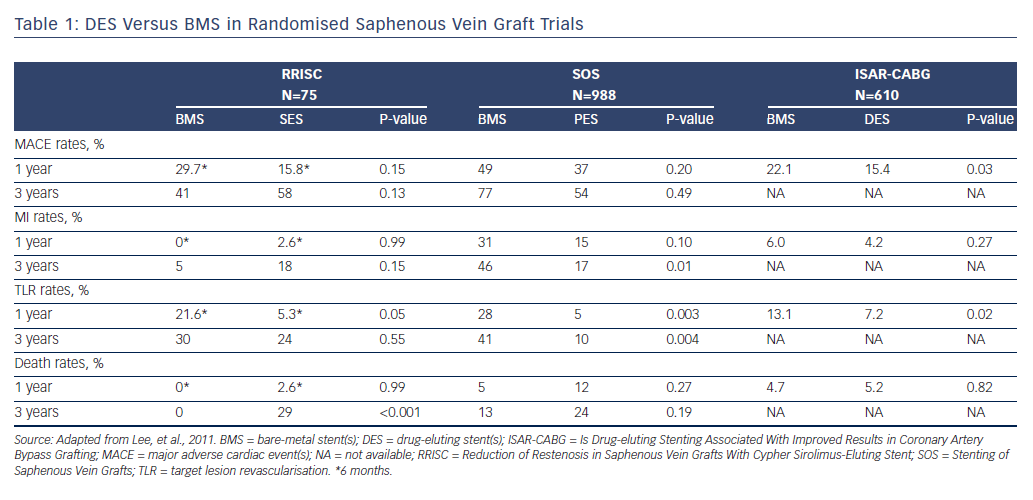
The Is Drug-eluting Stenting Associated With Improved Results in CABG (ISAR-CABG) trial, which randomised 610 patients with diseased SVG to DES or BMS, reported that DES were associated with lower rates of TVR (7.2 % versus 13.1 %; P=0.02) and met the primary endpoint of 1-year MACE (15.4 % versus 22.1 %; P=0.03) (see Table 1).51 The Stenting of Saphenous Vein Grafts (SOS) trial also demonstrated a significant reduction in MACE rates with paclitaxel-eluting stents (Taxus, Boston Scientific Corp.) compared with BMS,52 which was driven by lower TLR rates without increased MI or death through nearly 3-year follow-up.53 Sirolimus-eluting stents (SES; Cordis) were studied in the Reduction of Restenosis In Saphenous Vein Grafts With Cypher Sirolimus-eluting Stent (RRISC) trial, which demonstrated a reduction in TLR, TVR, binary restenosis rate and late stent loss in the DES group compared with the BMS group at 6 months.54 Conversely, the DELAYED RRISC study found the TVR benefit was lost at 3-year follow-up,55 and that the DES group had increased mortality rates. However, the study was not statistically powered for clinical outcomes such as mortality.
Multiple meta-analyses (including non-randomised studies) comparing DES with BMS in SVG intervention support the superiority of DES demonstrated in the randomised trials discussed above.15,56–63 One meta-analysis reported that 39,213 DES patients had lower rates of MACE (odds ratio [OR] 0.63; 95 % CI [0.54–0.74]; P<0.001), TVR (OR 0.70; 95 % CI [0.57–0.86]; P<0.001) and TLR (OR 0.64; 95 % CI [0.50–0.84]; P<0.01), with no difference in stent thrombosis (OR 0.90; 95 % CI [0.61–1.32]; P=0.58) compared with 26,461 BMS patients.15 These benefits persisted at 36-month follow-up.
Lee et al. compared SES and paclitaxel-eluting stents head to head in a multicentre analysis of 172 real-world patients undergoing SVG intervention and found nonsignificant differences in survival (HR 1.28; 95 % CI [0.39–4.25; P=0.69) and TVR (HR 2.54; 95 % CI [0.84–7.72; P=0.09).64 A 2014 study compared first-generation DES with secondgeneration everolimus-eluting stents (EES) in SVG PCI, reporting that EES showed no significant differences in MI, TVR or cardiac death following risk adjustment during 4-year follow-up.65 In summary, although there is no specified class recommendation for DES in SVG PCI, the 2011 American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Society for Cardiovascular Angiography and Interventions (ACCF/AHA/SCAI) guidelines do note a ‘preference’ for their use over BMS.66
Bioresorbable vascular scaffolds have not been adequately studied in SVG intervention and therefore cannot be recommended at this time. However, given the large diameter of the SVG, it can be considered in select situations (see Figure 1).
Embolic Protection Devices
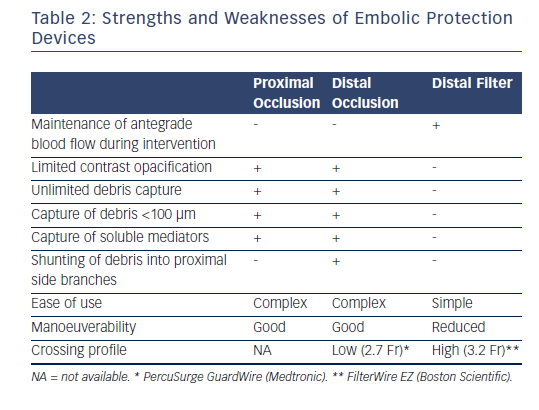
Embolic protection devices (EPD) were designed to capture and retrieve plaque particles that embolise during SVG intervention (see Table 2). In fact, MACE rates have been shown to double in SVG intervention compared with that of native coronary vessels.67 Despite the ACCF/AHA/SCAI class I indication for the use of EPDs during SVG intervention to decrease the risk of periprocedural MI, distal embolisation and no-reflow,66 they remain underutilized.68 EPDs can be categorised into distal occlusion aspiration devices, distal embolic filters and proximal occlusion aspiration devices.
Distal Occlusion Aspiration Devices
Distal occlusion aspiration devices use an interventional guidewire with an occlusion balloon that is inflated distal to the SVG lesion. Inflation obstructs antegrade flow, trapping plaque debris that is subsequently removed via an aspiration catheter. Such devices include the PercuSurge GuardWire (6F; Medtronic) and TriActiv® system (7F or 8F; Kensey Nash). The TriActiv includes a flush catheter for infusing heparinised saline during the procedure, which is absent in the GuardWire. Advantages of these EPDs include a low crossing profile and unlimited debris capture of particles <100 μm and soluble vasoactive mediators. Disadvantages include risk of embolisation during the wiring and device-crossing phase, ischaemia during balloon occlusion, limited contrast opacification and the risk of shunting debris into proximal side branches. Furthermore, guidewire selection cannot be tailored to procedural requirements and relatively disease-free distal landing zones are required.
The PercuSurge GuardWire became the first FDA-approved EPD following the results of the Saphenous vein graft Angioplasty Free of Emboli Randomized (SAFER) trial, which randomised 801 patients with SVG stenosis to stent placement over the GuardWire device shaft or a conventional angioplasty guidewire.69 EPDs significantly reduced the frequency of no-reflow (3 % versus 9 %; P=0.02), MI (8.6 % versus 14.7 %; P=0.008) and 30-day MACE (9.6 % versus 16.5 %; P=0.004). The TriActiv system was later FDA approved in the Protection During Saphenous Vein Graft Intervention to Prevent Distal Embolization (PRIDE) trial, which compared the TriActiv with both the GuardWire and the FilterWire EX™ (Boston Scientific) systems.70 TriActiv was non-inferior to the other devices in terms of 30-day MACE (11.2 % versus 10.1 %; P=0.65 [P=0.02 for non-inferiority]), but was associated with more vascular complications (10.9 % versus 5.4 %; P=0.01) and required more blood transfusions (7.7 % versus 3.5 %; P=0.02). These complications could be due to the larger calibre (8F) guiding catheters that were used in the TriActiv study arm.
Distal Embolic Filters
Distal embolic filters use filter bags with 100–110 μm-sized pores attached to the distal portion of a 0.014-inch guidewire with a delivery sheath. The filter bag is deployed distal to the target lesion to trap debris that embolises during the intervention and is later retrieved with its retained contents via a retrieval catheter. Advantages include the ability to maintain contrast opacification and perfusion during the procedure, along with ease of use. Disadvantages include the potential risk of distal embolisation during the wiring and device-crossing phases, debris embolisation during filter retrieval, inability to completely contain microparticles and soluble vasoreactive substances, large-diameter delivery sheath requirement and inability to deploy filters without a distal landing zone. Such devices include the FilterWire, Spider FXTM (Medtronic), Interceptor® PLUS Coronary Filter System (Medtronic Vascular) and CardioShield (MedNova).
The FilterWire EX became the first FDA-approved filter after completion of the FilterWire EX Randomized Evaluation (FIRE) trial, which compared the FilterWire EX with the GuardWire in 651 patients who received SVG PCI.71 The 30-day composite endpoint of MI, TVR or death was equivalent in both the FilterWire EX and GuardWire groups (9.9 % versus 11.6 %; superiority P=0.53, non-inferiority P<0.001), as were the 6-month MACE rates (19.3 % versus 21.9 %; P=0.44).72 The second-generation FilterWire EZ was then introduced with a lower crossing profile (3.2F versus 3.9F), smaller pore size (100 μm versus 110 μm) and overall improved delivery system compared with its predecessor. The Embolic Protection Transluminally with the FilterWire EZ Device in Saphenous Vein Grafts (BLAZE) registry reported that the success rate of this device was 97.8 %, and 30-day MACE rate was 6.7 % due entirely to non-Q-wave MI.73
The Spider Rx filtration device is also FDA approved for SVG intervention and was non-inferior to the FilterWire and GuardWire (MACE: 9.1 % versus 8.4 %; P=0.001 for non-inferiority) in the Saphenous Vein Graft protection In a Distal Embolic Protection Randomized Trial (SPIDER) trial.74 The Interceptor PLUS device was also non-inferior to the Filterwire and GuardWire in the (Assessment of the Medtronic AVE Interceptor Saphenous Vein Graft Filter System) AMEthyst trial.75 A multicentre, randomised clinical trial evaluating the CardioShield, a third-generation EPD, found the 30-day MACE primary endpoint occurred in 11.4 % with CardioShield versus 9.1 % with GuardWire (P=0.37), whereas intentionto- treat analysis demonstrated a strong trend for CardioShield noninferiority (P=0.57).76 A secondary modified intention-to-treat analysis including only patients receiving treatment device without protocol deviation also supported non-inferiority of CardioShield (P=0.022).76
Proximal Occlusion Aspiration Devices
The Proxis™ (7F; St. Jude Medical), which is no longer commercially available, is a proximal occlusion aspiration device that uses a guiding catheter with an inflatable balloon tip deployed proximal to the SVG lesion. This temporary suspension of antegrade flow generates a column of stagnant blood containing debris that is later aspirated via the guiding catheter. The balloon is deflated to restore antegrade perfusion following the intervention. Its advantages include use in lesions without a distal landing zone, retrieval of both atheromatous debris and vasoactive substances, protection from emboli prior to lesion crossing, protection of proximal side branches and the ability to tailor guidewire selection to procedural requirements. Notable disadvantages include limited contrast opacification and ischaemia during balloon occlusion. The Proximal Protection During Saphenous Vein Graft Intervention (PROXIMAL) trial evaluated the Proxis system in 594 patients undergoing stenting in 639 SVG lesions.77 The Proxis study arm was non-inferior to the control arm with distal EPDs (FilterWire or GuardWire) in the primary composite endpoint of MI, TVR or death at 30 days (10.0 % versus 9.2 %; P=0.0061).77
Recommendations
The trial data demonstrate the efficacy of all three EPD classes in minimising ischaemic complications. Its use must account for the degree of distal embolisation risk and the complexity of the coronary anatomy itself, especially when certain EPDs require distal landing zones. As indicated in the ACCF/AHA/SCAI guidelines, EPDs should ultimately be used during SVG intervention whenever feasible.66
Although these devices have proven effective during SVG intervention, they remain remarkably underutilised. An evaluation of 19,546 SVG PCI procedures in the American College of Cardiology-National Cardiovascular Data Registry found that EPDs were used in only 22 % of cases, despite being independently associated with a lower incidence of no-reflow (OR 0.68; P=0.032).78 One potential reason for this underutilisation could be that the delivery sheath heft makes distal filter deployment challenging. A recent study by Kaliyadan et al. highlighted the use of adjunct delivery techniques to optimize filter delivery in SVG procedures.79 Deployment failure in this study was reduced from 21.9 % initially to 7.6 % after using adjunct delivery techniques (P<0.01).79 Such techniques that facilitate device delivery success could potentially improve clinical outcomes and promote more frequent use of distal protection.
Adjunctive Pharmacology
Various pharmacological strategies can be used to decrease ischaemic complications during SVG intervention.
Glycoprotein IIb/IIIa Inhibitors
Adjunctive use of glycoprotein (GP) IIb/IIIa antagonists does not provide significant benefit in SVG intervention.80–82 The ACCF/AHA/SCAI guidelines recommend a class III (no benefit) indication for use of these agents in SVG lesions.66 The Evaluation of IIb/IIIa platelet receptor antagonist 7E3 in Preventing Ischemic Complications (EPIC) trial reported a reduction in the rate of distal embolisation in patients treated with GP IIb/IIIa inhibitors, but 30-day and 6-month clinical endpoints were comparable to those of the control group.80 Post hoc analysis of the FIRE trial showed a trend toward improved procedural success when GP IIb/IIIa inhibitors were used with filter-based embolic protection (P=0.058), but 30-day MACE rates were unchanged.83 Its potential benefit must be balanced by the potential risk of bleeding during SVG intervention.
Anticoagulants
Dual antiplatelet therapy recommendations for optimal treatment of SVG disease prior to hospitalisation are similar to that in native coronary vessel PCI.66,84 However, the ideal anticoagulants for SVG intervention have not been specifically established. A single-centre, retrospective, observational study reported that bivalirudin was associated with a significant reduction in major CK-MB level elevation compared with unfractionated heparin.85 Net clinical endpoints and rates of ischaemic bleeding were similar with bivalirudin monotherapy, bivalirudin plus a GP IIb/IIIa inhibitor and heparin plus a GP IIb/IIIa inhibitor in a subset of patients undergoing SVG intervention in the Acute Catheterization and Urgent Intervention Triage Strategy (ACUITY) trial.86 Bivalirudin alone had fewer minor bleeding complications compared with heparin plus a GP IIb/IIIa inhibitor (26 % versus 38 %; P=0.05). Heparin remains a popular choice for all forms of PCI, as the current ACCF/AHA/SCAI guidelines recommend a class I indication for its use in this setting.66
Vasodilators
Intragraft administration of vasodilators targets microvasculature to combat slow and no-reflow phenomena. Microcatheters can maximise pharmacotherapy delivery to these vessels. Pretreatment with intracoronary adenosine, a potent dilator of arteries and arterioles, decreases MI incidence after elective PCI,87,88 while it improves myocardial flow89,90 and lowers the incidence of no-reflow in the setting of acute MI.89,91 Adenosine may help reverse slow and no-reflow phenomena in patients undergoing SVG intervention.92,93 High doses of intragraft adenosine (at least five boluses of 24 μg each) significantly improved final Thrombolysis In Myocardial Infarction (TIMI) flow grade compared with low doses (less than five boluses) of adenosine (2.7 ± 0.6 versus 2.0 ± 0.8; P=0.04) and led to more slow and no-reflow reversal (91 % versus 33 %; P=0.02).93
Intragraft verapamil was effective in reducing no-reflow in SVG PCI.94–96 Intragraft verapamil (100–500 μg) improved flow in all 32 episodes of no-flow (TIMI flow grade 1.4 ± 0.8 pre- to 2.8 ± 0.5 post-intragraft verapamil; P<0.001) and re-established TIMI flow grade 3 in 88 % of cases.94 Prophylactic intragraft verapamil prior to SVG intervention mitigated occurrence of no-reflow compared with placebo (0 % versus 33.3 %; P=0.10) and increased TIMI frame count (53.3 ± 22.4 % faster versus 11.5 ± 38.9 %; P=0.016).96
Prophylactic intragraft nicardipine without the use of a distal protection device followed by direct stenting for degenerated SVG was shown to be safe and effective with low rates of slow-/no-reflow (2.4 %) and in-hospital MACE (4.4 %).97 Despite the lack of a control group for direct comparison, nicardipine appeared clinically beneficial compared with historical control data of SVG PCI procedures performed without nicardipine or distal protection devices.98,99 Nicardipine is not only used prophylactically during PCI, but is also safe and highly efficacious in reversing no-reflow, as demonstrated by Huang et al.100
Nitroprusside promotes nitric oxide production to induce vasodilation. One case-control study of patients who underwent SVG intervention pretreatment with nitroprusside (50–300 μg) reported significant reduction in periprocedural elevation of CK-MB levels >3x and >5x ULN, but no reduction in slow or no-reflow.101 However, another study found that nitroprusside (median dose 200 μg) injected into a diseased SVG led to highly significant and rapid improvement in both angiographic flow (P<0.01 compared with pretreatment angiogram) and blood flow velocity (P<0.01 compared with pretreatment angiogram) in SVG interventions complicated by either impaired flow or no-reflow.102
Conclusion
SVG conduit degeneration, restenosis and friable lesions with high embolic potential attenuate long-term CABG survival, while SVG intervention remains susceptible to high rates of periprocedural MI and no-reflow. When SVG disease requires intervention, proper stents, EPDs and pharmacological selection are essential for minimising complications. Both first- and second-generation DES demonstrate superiority over BMS in SVG intervention. The ACCF/AHA/SCAI guidelines recommend EPD use whenever feasible during SVG intervention to decrease the risk of embolisation complications. The optimal pharmacological treatment for slow or no-reflow is unclear, but various vasodilators show promise. When achievable, pan-arterial revascularisation or hybrid native coronary stenting with arterial revascularisation should be considered to minimise vein graft conduits in CABG.