Even with advancements in risk factor modification and improvements in care, stroke is the fifth leading cause of death in the US. Cerebrovascular disease kills 140,000 Americans each year, 795,000 people have a stroke each year and 87% of these strokes are ischaemic.1 The incidence of stroke in Europe varies from 101.1 per 100,000 to 239.3 per 100,000, with a higher incidence in eastern Europe and a lower incidence in southern Europe.2 About 1.4 million strokes occur each year in Europe, in a population of about 715 million. In Europe, stroke is the second leading cause of death after heart disease, causing 1.1 million deaths each year.3 Stroke incidence increases with age; the median age for stroke is 73 in Europe and 69 in the US. As the population continues to age, it is likely that stroke will become an issue vascular surgeons and specialists will need to address with increased frequency.
Risk Factors
There are a handful of risk factors for developing significant carotid artery disease: hypertension, diabetes, hyperlipidaemia and smoking. The Society for Vascular Surgery in the US and the European Society for Vascular Surgery recommend treating these risk factors medically and urge smoking cessation.4,5 Hypertension is an independent risk factor for stroke and it is compounded in the presence of diabetes. Recommendations are for long-term control of blood pressure with goals below 140/90 mmHg. Diabetes does lead to vessel wall changes, but strict glycaemic control has not shown to reduce the risk of stroke. However, it has been shown to reduce the risk of cardiovascular death, and so in patients with carotid artery disease, it remains a Grade 1C recommendation for people with diabetes who have a high-risk of coronary artery disease. In the Asymptomatic Carotid Surgery Trial (ACST), lipid-lowering therapy was shown to reduce the risk of stroke in both the surgical and medical arms.6 Evidence suggests the greatest benefit when LDL is aggressively lowered to 1.81 mmol/l or a greater than 50% reduction using high-dose statins.5 Smoking worsens all vascular disease and carotid artery disease is no different; even passive exposure to smoking can increase a person’s risk of stroke risk.7
Aspirin is recommended as antiplatelet therapy for all patients with carotid stenosis. Dual antiplatelet therapy has been shown to decrease the risk of recurrent stroke and should be considered in symptomatic patients.8 It has not been shown to increase the risk of moderate to severe bleeding, nor does it increase the risk of bleeding in patients undergoing carotid endarterectomy (CEA).9
Imaging
Carotid duplex remains the first-line imaging modality for identifying the presence and severity of carotid disease. As a low-cost, non-invasive test, it is easily accessible to most patients and physicians. Doppler flow velocities remain the mainstay for defining the extent of carotid artery stenosis. Using criteria established in the North American Symptomatic Carotid Endarterectomy Trial (NASCET), a peak systolic velocity (PSV) of ≥125 cm/s corresponds to a ≥50% stenosis and a PSV of ≥230 cm/s corresponds to a ≥70% stenosis.10 These measurements have been verified in subsequent studies and are used by both the Society for Vascular Surgery and European Society of Vascular Surgery.
Comparing internal carotid artery/common carotid artery diameter ratios and elevated end diastolic velocities allows providers to stratify 70-–79% and 80–89% stenoses but do little to aid in the sensitivity or specificity of detecting ≥50% and ≥70% stenosis.11
Computed tomography angiography (CTA) and magnetic resonance arteriography (MRA) can provide adjunctive information about plaque morphology and extra information obtained from carotid duplex. MRA is prone to overestimating the degree of carotid artery stenosis but does not require ionising radiation. This can make it difficult to use MRA results to determine whether a carotid stenosis is moderate or severe.12 It can provide information regarding plaque morphology such as the presence of a lipid-rich necrotic core and a fibrous capsule with high sensitivity and specificity.13 CTA does not fall prey to the same overestimation as MRA and can provide information about the characteristics and extent of the carotid plaque. It is less capable of elucidating the composition of the plaque, save for its detection of calcifications in the plaque and arteries. Both allow imaging of the aortic arch and common carotid arteries.
Carotid angiography uses contrast agents and carries the risk of stroke. As the most invasive testing method for carotid artery stenting (CAS), it is used the least. Current guidelines suggest only using it when other imaging modalities produce conflicting degrees of stenosis within the carotid artery. It may also be an appropriate imaging choice in patients with renal insufficiency, obesity or ferromagnetic devices precluding MRA or CTA.
Randomised Trials of Carotid Endarterectomy Versus Carotid Artery Stenting
Early studies were mixed in their procedural protocols and enrolment. The Endarectomy versus Angioplasty in patients with symptomatic severe carotid stenosis trial (EVA-3S) was a French trial in 527 patients.14 This study was terminated early because of an increased rate of death and stroke in the stenting arm, which tracked to 5-year follow-up but not to 10 years.14 The Stent-Protected Angioplasty versus Carotid Endarterectomy (SPACE) trial was a randomised trial that favoured CEA over CAS.15 It was terminated early secondary to enrolment issues. In the International Carotid Stenting Study (ICSS), 1,713 symptomatic patients with stenosis >50% were randomised between the two modalities.16 The 120-day incidence of stroke, MI or death was higher in the stenting group with the risks becoming similar at 5 years.16 Stenting and Angioplasty with Protection in Patients at High Risk for Endarterectomy (SAPPHIRE) randomised 334 high-risk patients to CAS with embolic protection device (EPD) versus CEA. The study’s short-term results favoured CAS, while long-term results showed no difference between the two modalities.17
All the trials except SAPPHIRE found CAS to be inferior, with higher rates of stroke and/or death when compared with CEA. Despite this, conclusions on the inferiority of CAS could not be drawn. Protocols among the studies were not uniform. Dual antiplatelet therapy, which is now standard treatment for CAS, was not mandated in EVA-3S or ICSS.14,16 Proximal occlusion or EPDs are also now standard treatment, but EPDs were not required in any of the studies.18 The Carotid Revascularization Endarterectomy versus Stenting Trial (CREST) was responsible for demonstrating the necessity of these while following specific protocols during CAS.18 CREST was a large, multicentre study that initially randomised symptomatic patients, but then included asymptomatic patients, to either CAS or CEA. It mandated stricter and more uniform procedural protocols (such as using an EPD) and closely trained and monitored clinicians involved in the trial. It found that while periprocedural stroke was higher with CAS, periprocedural MI was higher with CEA. It also found no difference in the rates of their primary endpoint and declared that CAS was not inferior to CEA. While this study has been criticised since its completion, the results have been used to construct guidelines recommending CAS even in asymptomatic patients.18
Meta-analyses of Randomised Controlled Trials and Reviews of Carotid Stenosis Data
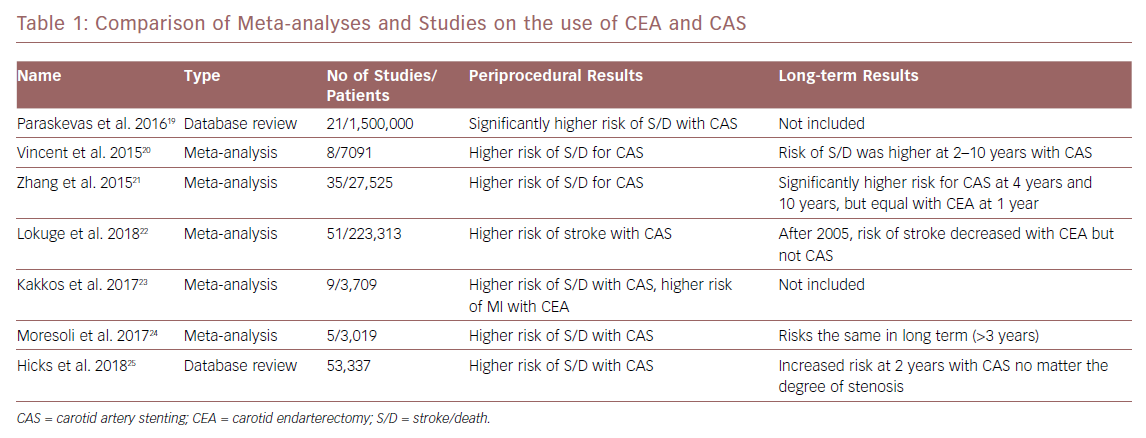
Since the publication of the CREST results and the increased use of CAS in symptomatic and asymptomatic patients, more randomised trials and comparison studies have been performed. Meta-analyses of these studies have shown the superiority of CEA in the short term, especially in the periprocedural period. CAS is associated with higher rates of stroke and death within 30 days while CEA showed only higher rates of MI and cranial nerve injury in the periprocedural period. However, when the long-term data (more than 2 years) from these studies is compared, some studies show the two treatment modalities appear to have similar outcomes when looking at stroke and death rates.19–24 Some newer data show a persistent higher rate of adverse events in CAS compared with CEA in asymptomatic patients when comparing these events in both a severe stenosis group (60–79%) and a very severe stenosis group (≥80%).25
A more recent meta-analysis shows that, despite heterogeneity of the studies, there has been a decrease in rates of complications from CEA over time.22 Since 2005, there has been a fall in rates of periprocedural complications from CEA while the rates of death and stroke from CAS remain largely unchanged. This has been seen in both symptomatic patients undergoing CEA (a decrease from 5.11% to 2.68% after 2005) and asymptomatic patients (3.17% to 1.50% after 2005).22 Readmission rates have found to be lower in those patients undergoing CAS; however, when those patients are readmitted, they can have stent complications that lead to stroke and death.26 Table 1 shows a comparison of the meta-analyses and data studies.
Current Trials of Asymptomatic Carotid Artery Stenosis
Given the lack of conclusive data on the use of CAS in asymptomatic patients, and given the advancements in both medical therapies and endovascular technologies, the debate over the use of CAS continues. Multiple trials are currently recruiting to determine the value of carotid revascularisation in the era of new medical therapies, as well as elucidating the updated risks of stroke and death with each method.
ACST-2 is an ongoing trial that plans to enrol 5,000 patients with asymptomatic carotid artery stenosis and randomise them to CAS and CEA. It has enrolled more than 2,000 patients and plans to release results in 2021. Early results show only a 1% rate of stroke, MI and death in all participants at 1 month.27,28
SPACE-2 began in 2008 as a three-arm trial randomising patients to BMT, CAS + BMT or CEA + BMT.29 In 2013, the trial was divided into two parallel trials of CEA + BMT versus BMT, and CAS + BMT versus BMT after initial enrolment goals were not met. It was halted after enrolling 513 patients over 5 years. It found no incidence of stroke or death in the BMT group at 30 days though this was the smallest group with the least significant stenoses. As a result, it is hard to fully interpret this study and apply it to current treatment guidelines. The rate of stroke in those undergoing CEA was 1.97% and 2.54% in those undergoing CAS.29
CREST-2 is a multicentre, randomised trial designed as two parallel trials. In one, BMT is being compared with CEA + BMT. In the other, BMT is being compared with CAS + BMT. The study began in 2014 and recruited asymptomatic patients. It plans to look at the incidence of stroke and death at 44 days post procedure with a 4-year follow-up. The goal is to enrol 2,480 patients with plans to release data in 2020. Interim results have not been released.30
European Carotid Surgery Trial-2 (ECST-2) is a large, randomised trial that plans to look at symptomatic and asymptomatic carotid artery stenosis >50% in patients with a 5-year risk of stroke <15%. It will randomise patients to optimal medical therapy (OMT) versus CEA + OMT and CAS versus OMT. In this, OMT is BMT with the addition of antiplatelet or anticoagulation. The trial began in 2012 with a goal to enrol 2,000 patients. As of May 2017, only 247 patients had been enrolled.31
Future Technologies
Silk Road Medical has designed a device for transcarotid artery revascularisation (TCAR) – the Enroute Neuroprotection and Stent System. The device uses the pressure gradient between the carotid circulation and the femoral vein to reverse the flow of blood during carotid artery stenting. Stenting and balloon angioplasty of the carotid artery stenosis is performed during reversal of blood flow. This reversal of flow is to prevent any distal embolisation while a filter catches any particulates before returning the blood to the venous circulation. Direct access to the common carotid artery is required via a cutdown while the venous aspect is placed percutaneously. Direct access to the common carotid artery avoids any potential embolic events from aortic arch manipulation. A prospective, multicentre trial of the device named the Safety and Efficacy Study for Reverse Flow Used During Carotid Artery Stenting Procedure (ROADSTER) trial had a 99% technical success rate with a 1.4% stroke rate.32 Stroke and death cumulative rates were 2.8% and there were no cranial nerve injuries. This initial study looked at 141 patients and a study is currently enrolling to examine results of the Enroute system in 1,000 patients.32
In the US in 2016, the Food and Drug Administration and the Centers for Medicare and Medicaid Services created the TCAR Surveillance Project to follow patients undergoing TCAR at hospitals participating in the Vascular Quality Initiative. The project includes information about the patient’s disease, the procedure and 1-year follow-up. A recent retrospective cohort analysed and compared 638 TCAR cases to 10,136 transfemoral carotid artery stenting (TFCAS) cases.33 In-hospital mortality was no different between the two groups, but in-hospital transient ischaemic attack (TIA)/stroke and the composite endpoint of TIA/stroke/death was higher in the TFCAS group compared with the TCAR group (3.3% versus 1.9% and 3.8% versus 2.2%, respectively). After multivariate comparison, the odds ratio for both in-hospital neurologic event and TIA/stroke/death in follow-up was 2.10 (95% CI [1.08–4.08]; p=0.3) in the TFCAS group regardless of whether the patient had symptomatic or asymptomatic disease. These preliminary findings in a real-world setting help reinforce the safety of TCAR. The completion of this study will help to further identify the role of this new technology.
Current Guidelines
The Society for Vascular Surgery’s guidelines for management of carotid disease was updated in 2011 and recommends the following.4
CEA should be the first-line treatment for most symptomatic patients with stenosis of 50–99% and asymptomatic patients with stenosis of 60–99%. The perioperative risk of stroke and death in asymptomatic patients must be <3% to ensure benefit for the patient. Patients should also have a life expectancy of 3–5 years.
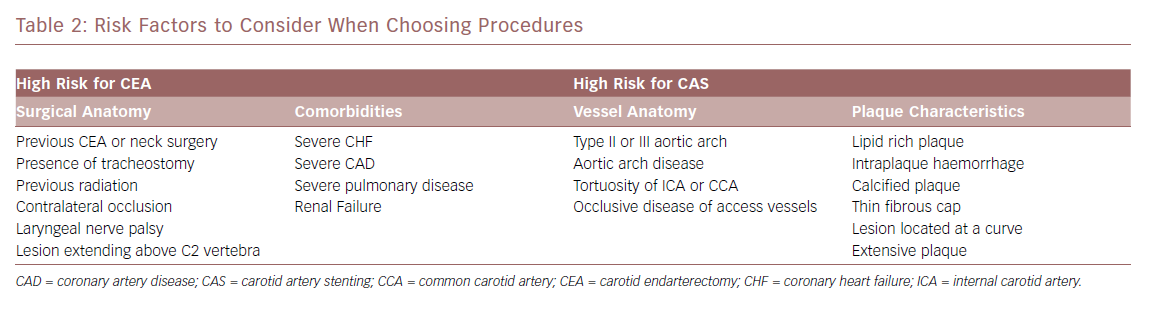
CAS should be reserved for symptomatic patients with stenosis of 50–99% at high risk for CEA for anatomic or medical reasons. These risks include uncorrectable and severe chronic obstructive pulmonary disease, congestive heart failure and/or uncorrectable coronary artery disease. Anatomic limitations include previous ipsilateral operation, tracheal stoma, external beam radiation to the area resulting in fibrosis or lesions proximal to the clavicle or beyond the vertebral body of C2.
CAS is not recommended for asymptomatic patients. While CREST demonstrates the equivalence of CAS in properly selected patients in the hands of experienced interventionalists, the widespread use of CAS in asymptomatic patients is not supported at this time.
Asymptomatic patients at high risk for intervention or with <3 years life expectancy should be considered for medical management as the first-line therapy.4
The European Society for Vascular Surgery updated its guidelines for carotid artery disease in 2017 and recommends the following.5
CEA should be considered in patients reporting carotid territory symptoms within the preceding 6 months and who have a 50–69% carotid stenosis, provided the documented procedural death/stroke rate is <6%.
In patients with average surgical risk and an asymptomatic 60–99% stenosis, CEA should be considered in the presence of one or more imaging characteristics that may be associated with an increased risk of late ipsilateral stroke, provided documented perioperative stroke/death rates are <3% and the patient’s life expectancy exceeds 5 years.
In average surgical risk patients with an asymptomatic 60–99% stenosis in the presence of one or more imaging characteristics that may be associated with an increased risk of late ipsilateral stroke, a CAS may be an alternative to CEA, provided documented perioperative stroke/death rates are <3% and the patient’s life expectancy exceeds 5 years.
CAS may be considered in selected asymptomatic patients who have been deemed by the multidisciplinary team to be high risk for surgery and who have an asymptomatic 60–99% stenosis in the presence of one or more imaging characteristics that may be associated with an increased risk of late ipsilateral stroke, and provided documented procedural risks are <3% and the patient’s life expectancy exceeds 5 years. Those criteria include silent infarction on CT, stenosis progression, large plaque area, plaque echolucency, intraplaque haemorrhage on MRI, spontaneous embolisation on transcranial Doppler and history of contralateral TIA.5
Deciding on a Treatment Modality
When evaluating a symptomatic patient, the route to determining the appropriate treatment is more straightforward. As long as the patient has an acceptable life expectancy, their anatomic and medical considerations are evaluated to decide whether a CEA or a CAS is the best option for the patient using previous guidelines. Each approach has specific circumstances where a complication is more likely to occur (Table 2). For CEA, the two main considerations are medical comorbidities and vessel anatomy, while plaque morphology and vessel anatomy play the largest roles for CAS. Earlier studies showed that age and renal failure placed the patient at higher risk of having complications after CEA. Recent studies, while small, show that age is less of a high-risk factor for CEA, suggesting that care for this group has improved.34 Renal failure with a estimated glomerular filtration rate <60 puts patients at risk of MI after CEA, even in recent data.35 Soft plaque and intraplaque haemorrhage have been shown to increase the risk of stroke during CAS. Early studies suggest that MRI analysis of the plaque before the intervention can allow better stratification of stroke risk during CAS.36,37 This knowledge can be used to be better stratify patients for the proper intervention.
For asymptomatic carotid stenosis, current evidence would suggest that CEA is preferable to CAS. While the advent of statins has improved the effect of BMT on asymptomatic carotid artery stenosis, the risk of stroke during observation of high-grade stenoses is still significant.38,39 There are also high-risk features for asymptomatic carotid artery stenosis that place patients at higher risk of having a stroke. These include progression of stenosis, unfavourable plaque appearance on ultrasound, silent infarcts on CT and reduced cerebrovascular reserve. Using these characteristics to stratify patients’ risk may further improve results of CEA.40,41 At present, CEA appears safer than CAS in the periprocedural period for asymptomatic carotid artery stenosis while results appear similar in the long term. Given the advent of new technologies and the lack of uniformity of previous studies, this difference in risk associated with each procedure may change.
Conclusion
Even with advancements in medical therapies and technologies, the management of carotid artery stenosis remains complicated. The optimal management for many patients is still not clear. It is likely that the focus will be on patient-specific therapies in the future. Current evidence advocates CEA for both symptomatic and asymptomatic carotid artery stenosis given the low incidence of periprocedural issues while CAS has a narrower range of indications. Ongoing studies have the potential to better clarify the risks associated with BMT, CEA and CAS while aiding the creation of new guidelines for the treatment of carotid artery stenosis. Despite the many published studies, more information is still needed regarding the best way to approach this complex problem.